Business
AI advisers could eventually guide consumers toward health products based on personalized needs rather than traditional marketing, IQVIA Consumer Health suggests. But brands will still need to balance data‑driven recommendations with the human appeal of in‑store discovery and self‑care experiences.
Umbrella branding in OTC medicines supports responsible self‑care by helping consumers navigate choices, build trust and confidence, and access reliable health information, countering regulators’ long‑standing focus on risks rather than benefits, says PAGB CEO Michelle Riddalls.
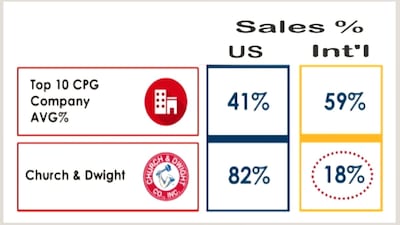
C&D starts 2026 with expectations to boost sales of latest acquisition, Touchland scented hand sanitizer, after halting sales of Flawless hair removal/nail care and Waterpik showerhead products and selling Spinbrush power toothbrush brand as well vitafusion and L’il Critter gummy supplement lines.
A round-up of the latest European people moves: Angelini hires external affairs chief; Pierre Fabre appoints impact and reputation officer; Boots changes leadership at the top.
Innovation & IP
AI advisers could eventually guide consumers toward health products based on personalized needs rather than traditional marketing, IQVIA Consumer Health suggests. But brands will still need to balance data‑driven recommendations with the human appeal of in‑store discovery and self‑care experiences.
Germany’s Expert Committee for Prescription (SVA) has unanimously rejected the proposed switch of the controversial painkiller metamizole from prescription to OTC status.
Futura Medical "delighted" with early feasibility study results for topical OTC treatment WSD4000.
Germany’s Expert Committee for Prescription meets on 20 January to discuss the Rx-to-OTC switch of controversial painkiller metamizole, which has been banned in a number of countries around the world because of the risk of agranulocytosis.
Policy & Regulation
Germany’s Expert Committee for Prescription (SVA) has unanimously rejected the proposed switch of the controversial painkiller metamizole from prescription to OTC status.
Recommendations to mitigate attrition among clinical research and drug manufacturing investigators at the FDA have not been implemented, and a Strategic Workforce Plan aimed at addressing recruiting, retention and training challenges has been shelved, the Government Accountability Office said.
Featuring: Prevention‑driven reforms in Vietnam; surging demand for OTC and herbal based interventions in China; Singapore updates its health data infrastructure; regional regulatory harmonization across Australia, Singapore and Indonesia; and WHO sets formal direction for TCIM.
Umbrella branding in OTC medicines supports responsible self‑care by helping consumers navigate choices, build trust and confidence, and access reliable health information, countering regulators’ long‑standing focus on risks rather than benefits, says PAGB CEO Michelle Riddalls.
Rx-to-OTC Switch
Germany’s Expert Committee for Prescription (SVA) has unanimously rejected the proposed switch of the controversial painkiller metamizole from prescription to OTC status.
Huge opportunities for OTC therapies loom in India, as consumerization brings structural reset in healthcare. Success stories on Rx-to-OTC transition augur well for pharma, but a predictable regulatory roadmap will be pivotal for sustainable growth.
In PDUFA reauthorization subgroup meetings, CHPA suggests expanding FDA’s special protocol assessment program to include OTC drug studies before FDA notes Congress has required it to provide switch-specific guidance before current PDUFA expires.
Germany’s Expert Committee for Prescription meets on 20 January to discuss the Rx-to-OTC switch of controversial painkiller metamizole, which has been banned in a number of countries around the world because of the risk of agranulocytosis.