Citeline, a Norstella company
News. Context. Impact.
In-depth journalism and analysis of the pharma, biotech, medtech, and consumer health industries.
Latest News & Analysis
The biopharma sector booked nearly the same number of acquisitions in each of the past two quarters, according to Evaluate data, but there are some encouraging signs in Q2.
Teva VP-global R&D and chief medical officer Eric Hughes talked to Scrip about building the company’s innovative pipeline in its growth-acceleration phase.
The creation of a single national formulary for medicines prescribing that could impact industry is among the action points in the UK government’s new 10-year health plan for the National Health Service in England.
With annual sales approaching $20m, LupinLife Consumer Healthcare has ambitious growth plans for the Indian OTC market.
DIA and BIO 2025 Highlights
Sponsors should investigate signals of possible fraud in a manner that does not raise alarms or else evidence might disappear, UK and US regulators said.
Biopharma executives and investors at the BIO convention noted the competition emerging from China with high-quality assets that could make strong additions to their portfolios.
Ongoing questions about user fees exerting too much influence over the FDA could spawn a shift in the PDUFA structure when talks begin later this year.
Financial markets were expected to loosen up in 2025, but BIO convention attendees indicated that conditions have worsened this year, so public and private firms are making tough choices.
ASCO 2025 Highlights
Deal Snapshot: The French drugmaker has been shifting its investments away from cancer to focus on immunology and inflammation but it continues to seek out interesting early-stage oncology assets.
The drugmaker presented data from its Phase I study of JNJ-79635322 at ASCO and EHA showing a 100% response rate in BCMA- and GPRC5D-naïve patients.
Antibody-drug conjugates are a major part of the oncology pipeline, and data presented at the American Society of Clinical Oncology meeting highlight the emergence of new technologies.
Innovent presented data on multiple pipeline assets at ASCO, including promising OS results for its first-in-class anti-PD-1/IL-2α-bias bispecific. The Chinese firm is also continuing to build out its metabolic disease portfolio and consider its partnering strategies, its CEO tells Scrip.
Explore Our Award-Winning Reporting
Immerse yourself in comprehensive global coverage, delivering unbiased, meticulously researched, and context-rich insights.

Regulatory & Policy
Essential updates and comprehensive coverage of industry regulations and emerging policy trends.
Commercial Insights
In-depth analysis of the global biopharma industry, offering news that goes beyond the headlines.

Executive Perspectives
Exclusive interviews, expert opinion, and critical insights tailored for life science decision-makers.

Medical Technology
Contextualized reporting on medtech advancements, market dynamics, industry data, and expert insights.
Off-Patent Medicines
Unrivalled news and analysis of the global generic, biosimilars and value-added medicines industries.

Health & Wellness
Your primary source for impactful news and insights in the health, beauty, and wellness industries.
Valuable Insights, Your Way

Engaging Articles
Engaging Articles
Quick to read yet rich in insight, our articles cover a diverse range of topics, delivering sharp, comprehensive analysis in every piece.

Contextualized News
Contextualized News
Go beyond the headlines with our in-depth news coverage, offering background and analysis to ensure you're fully informed on global events.
Future Trends Analysis
Future Trends Analysis
Discover emerging trends and innovative ideas with our forward-thinking thought-leadership providing actionable insights and visionary perspectives.
Thought-Provoking Podcasts
Thought-Provoking Podcasts
Listen to expert-led discussions and analysis through our engaging podcasts, designed to inform and inspire—whenever and wherever you are.
Timely Features
Timely Features
Stay up-to-date with our regularly updated features, delivering the latest trends, insights, and developments as they unfold.

Exclusive Conference Coverage
Exclusive Conference Coverage
Get insider access to major industry events with our detailed conference coverage, bringing you the latest insights from top leaders and innovators.
Unmatched Industry Coverage Year After Year
Access the full spectrum of our daily insights and in-depth reporting, delivering timely, actionable intelligence when it matters most.
7,000+In-Depth Articles
Expert insights delivered throughout the year500+Executive Interviews
Exclusive perspectives from industry leaders2,000+In-Depth Analyses
Deep dives into critical issues and trends500+Interactive Media
Engaging visual, audio and dynamic contentOur Expertise at a Glance
Discover how our dedicated team delivers unparalleled insights through rigorous research and expert analysis.
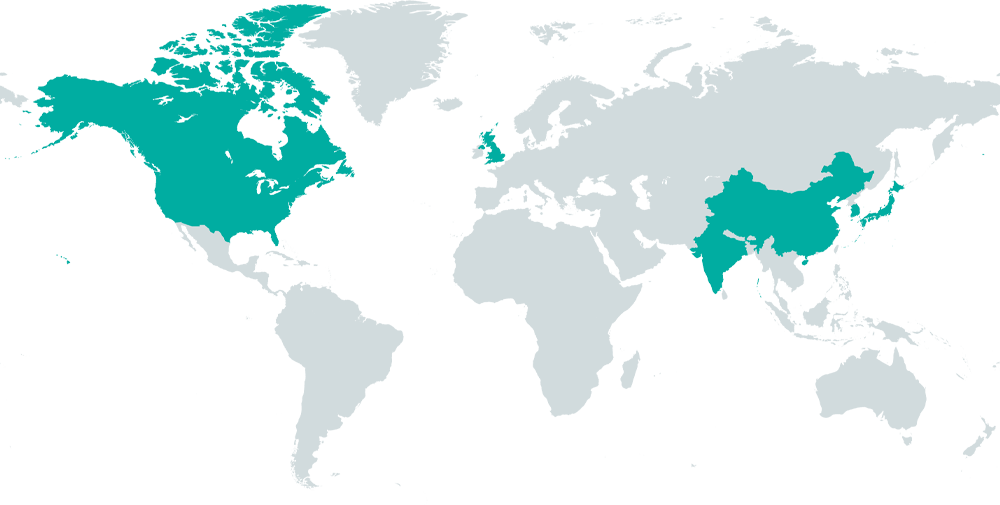
60+ Respected Journalists
Our team of over 60 trusted journalists operates across the US, Canada, UK, and Asia, delivering multilingual content.
Human Intelligence Filter
Local teams meticulously review industry news, filtering out 50-90% of the noise to highlight key events.

In-Person Meeting Coverage
Our journalists attend key events both in person and virtually, ensuring comprehensive coverage.

Thorough Research
We analyze regulatory and policy changes, check internal data, consult in-house analysts, review sell-side reports, and interview multiple sources.

Expert Interviews
We engage with company executives, clinical/medical KOLs, regulatory agencies, patient advocacy groups, payer organizations, lawyers, and other experts.

Premium News with Context
Our premium news includes in-depth analysis, insightful interviews, and comprehensive data.
In-Depth Analysis Powered by Citeline Data
Our global journalists leverage Citeline’s advanced analytics platforms to perform comprehensive data analysis and uncover valuable insights.
- Industry-Leading Market Analysis Tools: Comprehensive commercial, clinical, and regulatory insights.
- Real-Time Data Trends: Up-to-the-minute data for the latest industry developments.
- AI-Driven Solutions: Innovative technology accelerating drug development and delivery to patients.
- Custom Insights with Ask-the-Analyst: Tailored answers and analysis to address your specific questions.
These powerful tools deliver a wealth of information—from detailed industry reports to real-time data trends—enabling reporting that sets us apart from the rest.
Expert Analysis Across The Decades
With a rich history and commitment to excellence our seasoned journalists have consistently provided crucial industry insights, reflecting years of dedicated expertise.

Generics Bulletin
News and Expert Analysis on Generics and Biosimilars
In Vivo
Strategic Analysis for Medtech and Pharma Leaders
HBW Insight
News and Expert Analysis on the Healthcare Industry
Medtech Insight
News and Expert Analysis for Medtech Decision Makers
Scrip
Pharma News and Expert Analysis for Commercial Decision Makers
Pink Sheet
News and Expert Analysis on Pharma Policy and Regulation
US FDA
Beginnings of
today's FDA
Meet The Team

Karen Coleman
Executive Director,
News and Insights

Ian Haydock
Editor-in-Chief,
APAC

Nielsen Hobbs
Editor-in-Chief,
Pink Sheet

Eleanor Malone
Editor-in-Chief,
Commercial Insights

Andrea Charles
Vice President
Custom Content