Animal Testing
A new report identifies opportunities to implement the 3Rs principle in India, calling for a waiver of redundant animal testing for generics and NCEs/NBEs and alignment with global regulatory frameworks. Will India shift gears to adopt harmonized NAM-first policies?
Inductive Bio receives $21m from ARPA-H to develop AI toxicity models using human tissues, partnering with Amgen to replace animal testing in drug development.
Texas Democrat Lizzie Fletcher didn’t pose questions for witnesses at House Health Subcommittee hearing but used her floor time to question whether Congress will keep federal agencies afloat. “I have real concerns that even if we reauthorize these programs, we won't have the funding or the staff to
The European Medicines Agency, like its counterpart in the US, is increasingly focusing on the use of alternatives to animal testing.
A new EU public-private initiative is developing tools to help clarify when mini- and micropig models can be used as viable alternatives to non-human primates in non-clinical drug safety testing.
This is the second of a two-part profile of Hans Clevers, head of Pharma Research and Early Development at Roche, in which he talks about his vision for the future of personalized medicine.
General chapters on pyrogens, histamine and depressor substances – involving tests on rabbits, guinea pigs and cats – are being removed from the Ph. Eur, marking another step in the European Pharmacopoeia Commission’s ongoing efforts to replace, reduce and refine the use of animals for monograph requirements.
This is Part 1 of a 2-part profile of Hans Clevers, Head of Pharma Research and Early Development at Roche.
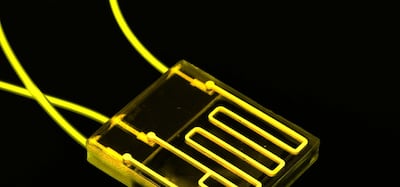
New alternative methods to animal testing in early drug development are increasing in sophistication and predictability, yet they are vastly underutilized. In Vivo presents an overview of select technologies and vendors.
Slow adoption of alternatives to animal testing in the current decentralized regulatory framework.
This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.
Despite increased openness by regulators and technological progress, the adoption of alternatives to animal testing remains challenging. The need for data validation by agencies and companies is a big factor.