Coronavirus COVID-19
It wasn’t a quick decision for Xlear to accept DoJ attorneys’ offer to dismiss the case. “I want the I want the FTC to change its policy and its behaviors,” says Xlear president Nathan Jones.
New product launches come with great expectations, which are sometimes unrealistic. This can lead to disappointment. Lately, market access restrictions are exacerbating sales challenges.
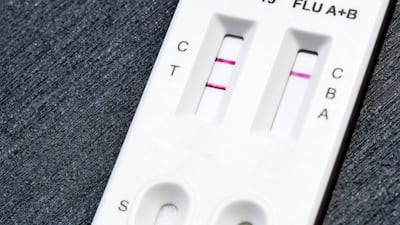
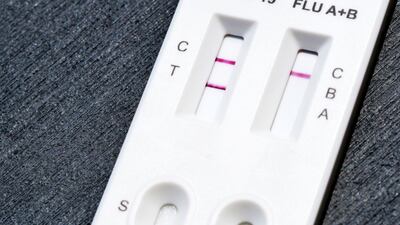
FDA grants de novo authorization to Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first OTC flu test to be cleared outside the emergency use pathway.
The US Food and Drug Administration has granted a de novo authorization to the Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first over-the-counter flu test to be cleared outside the emergency use pathway.
The US FDA is finalizing a guidance intended to encourage the use of decentralized clinical trial techniques. After the COVID-19 pandemic necessitated adoption of the more patient-friendly approaches, the agency is clearly eager to see them become routine.
The owner of a Chicago COVID-19 testing lab plead guilty to wire fraud for billing the government for COVID-19 tests that were not performed. Also, test developer Talis Biomedical agreed to pay $32.5m to settle a shareholder suit.
Elevated levels of the COVID-19 virus in wastewater samples have prompted the Biden-Harris administration to dust off its free testing program in preparation for the fall and winter. The US Postal Service will begin shipping the testing kits in September.
Pfizer/BioNTech and Moderna rapidly adapted Comirnaty and Spikevax for the 2024-2025 season to address the KP.2 variant after the US FDA had advised them to target JN.1 in June.
The companies said their combination mRNA vaccine showed protection against SARS-CoV-2 and influenza A, but not influenza B.
The FDA designated class II special controls classifications for two diagnostics and force separation catheters.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
Targeting the JN.1 subvariant of the SARS-CoV-2 virus will facilitate timely vaccination campaigns in Europe to help reduce the burden of disease associated with COVID-19, according to the EU regulator.