Cosmetic Ingredients
Mark Meador most recently worked as a visiting fellow at conservative think tank Heritage Foundation’s Tech Policy Center and worked during Trump’s first term as a trial attorney in the Department of Justice’s Antitrust Division. The two Democrat-appointee seats remain open while the members Trump recently fired contest his controversial decision.
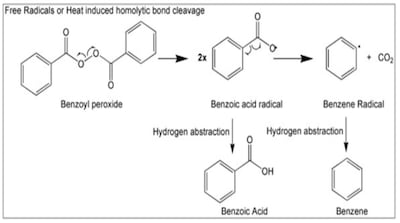
FDA testing of 95 benzoyl peroxide products due to concerns about elevated benzene detected by third-party testers found 90% with undetectable or extremely low benzene levels.
Supplies from Mexico and Canada could become even more important to US firms as well as more reliable than their current systems, says Rob Handfield, Bank of America distinguished professor of supply chain management at NC State.
The Washington State Department of Ecology will try to work with companies that violate the Toxic Free Cosmetics Act, rather than reflexively imposing the $5,000-per-violation fine for first-time offenders, says the law’s implementation planner. She noted financial assistance is available for small businesses, as well as incentives for companies adopting measures “beyond compliance.”
Toxic-Free Cosmetics Act planner Shari Franjevic acknowledges the Washington State Department of Ecology is authorized to use enforcement discretion regarding the new law’s 1ppm limit on trace lead in cosmetic products, effective 1 January 2025, or raise the ceiling via rulemaking. But it does not seem inclined to do either based on data known to the department at present.
Cosmetics Europe’s John Chave highlights the important role that industry must have in educating the public about chemicals used in cosmetic products. The association’s COSMILE Europe database launched last year is one such measure to combat misinformation while industry braces for potential impacts resulting from new hazard categories under the Classification, Labeling and Packaging (CLP) regulation.
The RAC opinion begins the process of prohibiting use of talc in cosmetic products marketed in the EU. A consultation period will follow the Commission’s preparation of a delegated act.
Mentholatum says two line extensions made with 2% salicylic acid and its "Oxy for Every Kind of Ne" campaign reinforce the brand’s “commitment to tackling every type of acne—from face-ne and chin-ne to body-ne.”
The Fragrance Creators Association president and CEO discusses the group’s vision for fragrance industry stewardship and opportunities provided by AI, biotechnology and wellness trends in an exclusive Q&A with HBW Insight.
Marking a first for the iconic brand, Eucerin Face is rolling out in Target doors now, Beiersdorf leaders noted in a 7 August half-year earnings presentation. The firm’s Consumer business segment recorded nominal sales growth of 5.4% to €4.3bn in the first half of 2024, led by Nivea.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
In addition to sustainability, Myers noted DEI and advancing alternatives to animal testing among key priorities for the group. “We have member companies that really lead the way on many of those. We want to be able to amplify their good work and their messaging, because they are good corporate citizens” and “it’s what consumers are demanding anyway,” he said in an interview.