Deals
A biosimilar collaboration between Kashiv and Saya aims to expand access to affordable supportive oncology therapies in Mexico and 10 additional Central America and Caribbean markets.
The antibody-drug conjugate pipeline has more than doubled to 895 candidates since 2023, with DNA topoisomerase I overtaking HER2 as the dominant target.
With another settlement secured, US states are gearing up for the first trial of the price-fixing case later this year.
During Q4, biopharmas brought in an aggregate $30bn in financing and device company fundraising totaled $9.7bn; while in vitro diagnostic firms and research tools players raised $1.5bn.
SK bioscience jumps into RSV preventive antibody space through new Gates alliance.
BC Partners has completed its acquisition from Servier of French generics giant Biogaran after the French government allowed the private-equity takeover on condition that BPI France take a minority stake. Meanwhile, former Biogaran CEO Erick Roche has returned to the role after a long stint at Teva.
Just a few days after Alvotech announced global settlements for its Eylea rival, Samsung Bioepis has followed up with its own agreements with the originators.
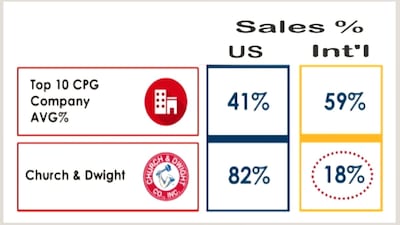
C&D starts 2026 with expectations to boost sales of latest acquisition, Touchland scented hand sanitizer, after halting sales of Flawless hair removal/nail care and Waterpik showerhead products and selling Spinbrush power toothbrush brand as well vitafusion and L’il Critter gummy supplement lines.
Sandoz and Alvotech have struck a deal that will see the firms collaborate on multiple biosimilar candidates for the Australian, Canadian and New Zealand markets across therapeutic areas including ophthalmology, immunology and gastroenterology.
Sunshine Pharma plans to invest at least $14m to set up a joint venture with fellow Chinese firm XtalPi, with goals including the discovery and development of novel molecules for autoimmune disorders.
UK major will bag CSPC’s eight preclinical drug candidates in weight management, including most advanced asset SYH2082, a long-acting GLP-1/GIP receptor agonist with once-monthly dosing potential.
Both companies announced that during special meetings on Jan. 29, their shareholders voted nearly unanimous support for Kimberly-Clark’s $48.7bn acquisition proposed in November at $3.50 per share plus 0.14625 share for each Kenvue share.