Dental Oral
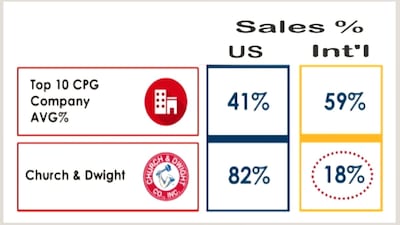
C&D starts 2026 with expectations to boost sales of latest acquisition, Touchland scented hand sanitizer, after halting sales of Flawless hair removal/nail care and Waterpik showerhead products and selling Spinbrush power toothbrush brand as well vitafusion and L’il Critter gummy supplement lines.
Dental guards already accounted for “well over half of the brand’s revenue” and DenTek claimed more than 50% of US category share when PCH launched "Fantasy Guards" campaign targeting fantasy football league enthusiasts.
P&G provides reasonable basis in NAD review for Crest 3D Whitestrips claims on labeling featuring numbers from 4 to 34 to indicate the “Levels Whiter” users’ teeth will appear. NAD says GuruNanda’s population in survey contesting P&G claims was “a fatal flaw.”
P&G supports brand and performance claims for Crest Pro-Health Detoxify toothpaste, NAD concludes, but will modify a fluoride claim following review on a challenge by GuruNanda.
P&G provided sufficient support for all challenged claims, including the “Gum Detoxify” brand used on the product label and in advertising on the website and in magazine detail pages.
This week, Establishment Labs Holdings announced the FDA gave it premarket approval for Motiva breast implant, Cologuard lands FDA approval for Cologuard Plus and GE HealthCare gets FDA nod for a new imaging agent. The FDA announces another expansion for TAP into ophthalmology and radiology. The AAMI and CTA will join forces to develop standards for AI and ML-enabled health care products.
Guidance documents issued 30 September explain how makers of air powered dental handpieces and air motors, dental cements, dental ceramics, and dental impression materials can bring their products to market by demonstrating compliance with established criteria, without a direct comparison to a predicate device.
The US FDA released six more device classifications in early September, including products from Edwards, Interscope, and Baxter Healthcare.
Laifen, Opalescence Moon, Lewie and quip are among US companies with recent launches to boost their oral care market shares. Recent launch by Arm & Hammer global oral care brand also targets driving growth in the category.
HiSmile appeals NAD recommendation following review on P&G challenge to discontinue ad claims on its website and in social media videos stating peroxide-containing whitening products are “painful” or cause pain, break down and impact gums and teeth, or “damage” gums.
While the latest missions from NASA may seem like the stuff of science fiction, discoveries from outer space are not only unlocking the mysteries of the cosmos, but improving technologies used every day on Earth, including those in the medtech industry.
National Advertising Division recommends Oral Essentials discontinue claims on packaging that Lumineux strips are "first on the market that are delicious and clinically proven to whiten teeth as well as the leading brand, without the harm associated with bleaches.”