Diagnostic Imaging
Atul Gupta, MD and CMO of Royal Philips’ Diagnosis & Treatment business, shares his views about potential developments in imaging and wider medtech in 2026 and the value of AI. He warns that clinicians will not adopt AI they don’t trust, understand or find helpful in real-world conditions.
Atul Gupta, MD and CMO of Royal Philips’ Diagnosis & Treatment business, shares his views about potential developments in imaging and wider medtech in 2026 and the value of AI. He warns that clinicians will not adopt AI they don’t trust, understand or find helpful in real-world conditions.
During his 40 years at Royal Philips, Bert van Meurs has seen the medical devices business grow in line with the evolving needs of healthcare providers with whom the company partners in customer and patient focused innovation.
During his 40 years at Royal Philips, Bert van Meurs has seen the medical devices business grow in line with the evolving needs of healthcare providers with whom the company partners in customer and patient focused innovation.
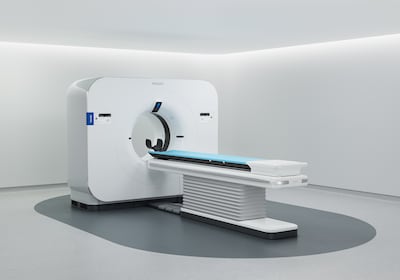
Verida optimizes the entire imaging chain with AI, lowering system noise, elevating image quality and accelerating clinical workflow and marks a transformative milestone in CT, Philips claims.
Chinese medtechs are expanding rapidly worldwide, but gaining market access within China itself – poised to become the world’s largest medtech market – is proving to be a very different challenge.
Cloud services accounted for 85% of Sectra’s imaging IT solutions business in 2024-25, and the Swedish medical diagnostic imaging and IT for precision medicine company is further expanding its digital healthcare and AI coverage in the UK, wider Europe and US.
Point of care ultrasound technology that can be used by non-sonographers will plug gaps in workforce coverage and bring new critical care capabilities to the frontline of care, said Royal Philips.
Bringing AI and digital healthtech innovation to patients works most efficiently via a three-way partnership in which clinicians and cloud services have equal weighting with a powerhouse innovator, Royal Philips told In Vivo during ECR 2025.
Philips, the last of the global top-three imaging companies to report 2024 financial results, said the soft China market put the brakes on its growth. The regional outlook for early 2025 is no different, and US-China tariffs will present another challenge.
The US market’s largest imaging manufacturer marked two years as an independent company with plans to invest in more M&A and PDx production capacity, but reported a modest 2025 revenues outlook.
Any tariff headwinds should be counterbalanced by currency tailwinds, predicts German imaging and diagnostics company Siemens Healthineers.