Diagnostics
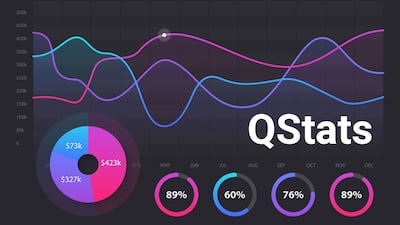
During Q4, biopharmas brought in an aggregate $30bn in financing and device company fundraising totaled $9.7bn; while in vitro diagnostic firms and research tools players raised $1.5bn.
With capital being recycled, tariffs settling and interest rates softening, 2026 could provide more investment and growth opportunities in pharma and medtech, with AI giving a tailwind, say Taylor Wessing’s Ross McNaughton and Sarah Cole.
The medtech industry has been disrupted by tariffs and China’s volume-based procurement policies. While India is expected to emerge as a key long-term growth market, near-term upside may come from a potential rebound in EU attractiveness following the MDR reform proposals in December 2025.
With capital being recycled, tariffs settling and interest rates softening, 2026 could provide more investment and growth opportunities in pharma and medtech, with AI giving a tailwind, say Taylor Wessing’s Ross McNaughton and Sarah Cole.
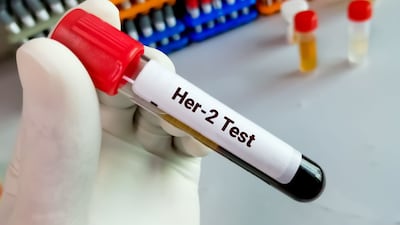
Enhertu's approval for ever-lower levels of HER2 expression is pushing standard immunohistochemistry assays beyond their limits. Pathologists say they have a solution.
The rise and fall of US tariffs and the chill headwind from China’s state procurement policies have blown onto medtech’s radar. But how to monetize AI is the dominant concern up and down the industry.
The rise and fall of US tariffs and the chill headwind from China’s state procurement policies have blown onto medtech’s radar. But how to monetize AI is the dominant concern up and down the industry.
In this special In Vivo podcast episode, executive editor Lucie Ellis-Taitt is joined by an expert panel of Citeline journalists – Ashley Yeo, David Wild, Jessica Merrill and data journalist Edwin Elmhirst – to explore the trends set to reshape the biopharma and medtech sectors as we head into 2026.
Brain-computer interfaces advance toward trials and commercialization, Oura pushes for FDA-cleared blood pressure monitoring, and regulators weigh AI’s expanding role in mental health and diagnostics amid rising safety concerns.
During Q3, biopharma merger and acquisition deal value reached $40.7bn and drew in $66.5bn in potential deal value from alliances. Device company M&A values reached $6.4bn, while in vitro diagnostics and research tools players’ M&A activity totaled $15m.
During Q3, biopharmas brought in an aggregate $20.1bn in financing and device company fundraising totaled $2.5bn; while in vitro diagnostic firms and research tools players raised $1.2bn.
June Raine was a “hard act to follow” at MHRA, but new chief executive Lawrence Tallon is looking to the future in setting out a vision for the UK regulator.