Drug Approval Standards
“Our sense is that companies are currently focused on timing, scope and execution. For example, knowing when a final order might issue … We also imagine that formulators are scrutinizing the proposed list of permitted combinations, the 6% cap, and the permitted dosage forms,” say ArentFox Schiff att
Agency says FY 2026 Tier 1 OMOR fees are $587,529 and Tier 2 fees are $117,505. The fees, due to FDA when an OMOR is filed, aren’t included in OMUFA target revenue calculation for each fiscal year based entirely on annual facility registration fees.
CDER Office of Generic Drugs publishes MaPP for prescription-to-nonprescription switches and ANDAs to explain regulatory responsibilities for makers of generic copies of reference listed drugs approved for OTC switch.
Sunscreen products industry and public health advocacy groups have been critical that FDA has not approved a new filter since 1999 even as countries in Europe and other regions allow using numerous additional ingredients in sunscreens.
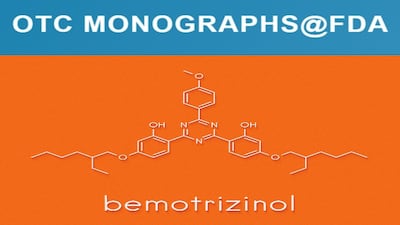
FDA’s proposed order follows its review of OMOR DSM-Firmenich submitted showing bemotrizinol, at concentrations up to 6%, is generally recognized as safe and effective and can be added as an active ingredient to sunscreen monograph.
CDER Office of Generic Drugs publishes MaPP for prescription-to-nonprescription switches and ANDAs to explain regulatory responsibilities for makers of generic copies of reference listed drugs approved for OTC switch.
FDA says Teresa Michele, in CDER nonprescription drugs program leadership roles since 2013, was being moved to another position in the agency.
In announcement of approving VKT Pharma’s application, FDA included storage and handling instructions common in labeling, suggesting concerns persist about the potential for NDMA to form after products are distributed.
In announcement of approving VKT Pharma’s application, FDA included storage and handling instructions common in labeling, suggesting concerns persist about the potential for NDMA to form after products are distributed.
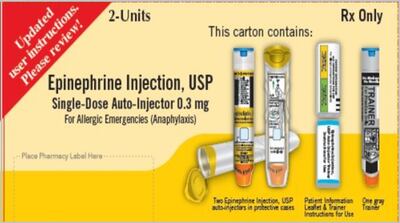
Approval of numerous Rx formulations hasn’t translated to wider availability when a remedy is needed for anaphylaxis emergency, say FDA and the Margolis Institute in announcing workshop on reducing anaphylaxis-related morbidity and mortality.
OMUFA reauthorization and directions for FDA to provide reports on monograph program and guidance on improving chances for OTC switch applications included in recent stopgap spending bill to end federal government shutdown.
Brazil’s medicines regulator, ANVISA, has hired new staff to help halve the time it takes to register a new medicine in Brazil.