ESMO
Data presented at the Berlin meeting highlight the potential of the company's cell therapy anzu-cel to redefine the treatment paradigm for uveal melanoma.
In a China-only Phase III trial, Sichuan Kelun-Biotech’s sac-TMT significantly cut the risk of death by 40% versus chemotherapy in the second-line treatment of non-squamous EGFR-mutated NSCLC. The ADC was administered at a higher dose than in Merck’s multiple Phase III trials of the same agent.
The company presented detailed data at ESMO and said its Phase III trial might have reached statistical significance with a larger sample size.
The company breaks new ground with Keytruda for platinum-resistant ovarian cancer and unveils promising Phase II data on its Daiichi-Sankyo-partnered ADC raludotatug deruxtecan.
Data from the EV-303/KEYNOTE-905 trial show the potential for the combination of Astellas/Pfizer’s ADC and Merck’s PD1 blocker to redefine standard of care.
Lupin’s poster presentation at the upcoming ESMO Congress is set to highlight that its STING agonist candidate demonstrated safety in a Phase Ia trial. The question is whether it will be able to strike a deal like IFM Therapeutics did with Novartis or Mersana did with Merck KGaA
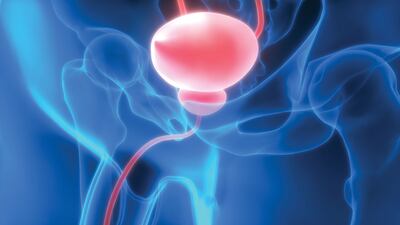
J&J acquired Taris in 2019 to gain access to TAR-200 and its drug delivery platform. Now, early clinical data suggest the product could delay or remove the need for cystectomy in several bladder cancer settings and the US healthcare giant is eyeing multi-blockbuster sales.
The Barcelona meeting saw early data from two candidates from Incyte and Pfizer which suggest some progress for a new drug mechanism that has so far underwhelmed.
The company’s investment in oncology is starting to pay off, with its PD-L1/VEGF inhibitor BNT324 generating particular interest at ESMO.
The German group's goal of expanding Nubeqa into wider prostate cancer patient populations has probably been reached with the results from the Phase III ARANOTE study, while a combination of its older product Xofigo and Pfizer and Astellas’ Xtandi is set to become standard of care for another subset.
New Phase II data for the bispecific antibody show promise in colorectal cancer as the company seeks to spread the bispecific antibody’s wings. Phase III trials are on the horizon, where it will need to go up against standard of care.
Pfizer will talk to regulators about pivotal study designs in cachexia following promising ponsegromab Phase II data, with results from a separate study in heart failure also coming soon.