In Vitro Diagnostics
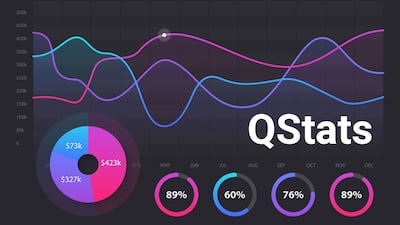
During Q4, biopharmas brought in an aggregate $30bn in financing and device company fundraising totaled $9.7bn; while in vitro diagnostic firms and research tools players raised $1.5bn.
If medtech regulation is to work for patients, industry and society, Europe must move from political positioning to evidence-driven policymaking. It is time to avoid repeating the self-destructive cycle.
AI promises to be the much-needed solution to the now overly complex field of EU medtech regulatory affairs. Will every stakeholder start using solutions such as Raiana?
Abbott started 2026 as it ended 2025 – with major regulatory breakthroughs for pulsed field ablation innovations, boosting its EP credentials in the US and EU. It also broadened its IVDs platform by adding cancer detection and screening company Exact Sciences in a deal expected to close in Q2.
The European Commission’s proposal does not envisage a single centralized medtech agency, but instead sets out a targeted redistribution of tasks that could strengthen the existing framework. Professor Tom Melvin told Medtech Insight how.
Could the understaffing that has weighted down EU’s medtech regulators become a thing of the past?
European Commission-sponsored study published in December reveals the full impact of soaring compliance costs, shrinking device portfolios and innovation shifting abroad.
Proposal Gives Unrealistic Timelines And Reflects Poor Grasp Of Notified Body Realities
There are many unworkable suggestions in the European Commission’s proposed overhaul of how notified bodies operate, TEAM-NB’s document states
MedTech Europe’s Petra Zoellner discusses industry's response to EU regulatory proposals aimed at improving notified body operations, transparency, and cost predictability, ultimately fostering innovation in medical technology.
Industry associations support the commission’s MDR/IVDR revision simplification objective and aim to increase Europe’s competitiveness, but MedTech Europe also sees further room for improvement.
The just published proposed targeted revision of the Medical Device and IVD Regulations is a radical rethink of how the EU should regulate medical devices. The document is aimed at supporting industry and patients alike.