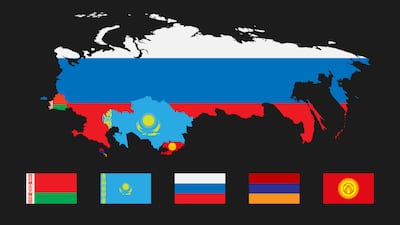
Kazakhstan
Country
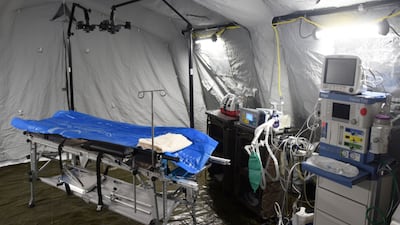
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have since stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
Ukraine’s medical supply needs have evolved since Russia launched its military invasion of the country on 24 February. EU healthcare decision makers and the medical technology industry have stood firm in their support of the besieged country, said MedTech Europe’s Jesús Rueda.
After a five-year regulatory transition, access to the Eurasian Economic Union market for new medtech products must now take place via the EAEU system only. But having got off to a slow start, further system transitional measures are now under discussion.
The Eurasian Union’s medical device regulatory system, many years in the planning, becomes mandatory in January 2022. But new amendments posted in late summer allow for national registrations to remain valid after the deadline. Moscow-based consultancy RegMT explains the background, and the likely path ahead.
There are just 4.5 months left until the new harmonized Eurasian Economic Union medtech system becomes mandatory, signifying major changes in regional market access. EAEU member Russia is meanwhile strengthening its post-market medtech controls.
The EMA has issued updated safety advice on AstraZeneca’s COVID-19 vaccine, Vaxzevria, Valneva has decided to pursue vaccine supply deals with individual member states after a falling out with the European Commission, and Kazakhstan has begun administering the first doses of its domestically produced vaccine, QazVac.
Kazakhstan’s pharmaceutical market showed positive dynamics in 2020. In the first 9 months of last year, market size for finished pharmaceutical products grew to KZT460bn($1bn), up 22% year-on-year.
The Medicines Patent Pool is giving interested parties until 18 December to submit license applications for the HIV treatment dolutegravir in four middle-income countries, following announcement of the agreement last month.
Dr Reddy's is poised to expand its OTC offering in Russia and certain CIS countries by snapping up a basket of allergy brands from fellow Indian firm Glenmark.
Reporting double-digit growth in the first half of 2019, Krka says it has mined marketing opportunities in new eastern European markets by introducing its established treatments for cardiovascular diseases
Russia and Kazakhstan, two prominent members of the Eurasian Economic Union, are continuing to build national medtech regulatory infrastructures, in the shadow of the forthcoming harmonized EAEU system, of which a postponement beyond 2021 has not been ruled out.
British probiotic specialist OptiBiotix Health has struck a three-year distribution deal for its CholBiome supplement in Russia and Kazakhstan.