Liver & Hepatic
The European Medicines Agency is set to adopt opinions on whether marketing approval should be granted to five new products, including therapies for a serious liver disease and an ultra‑rare genetic mitochondrial disorder.
AriBio reaches an exclusive global out-licensing deal with Restari for its next generation PDE-5 inhibitor.
The deal marks the third in the FGF21 analog space in the past five months.
The Alfasigma subsidiary will voluntarily take Ocaliva off the market after a request from the US FDA, which put Intercept’s trials of the primary biliary cholangitis drug on a clinical hold.
Zydus's saroglitazar beat both Gilead’s Livdelzi and Ipsen’s Iqirvo on biochemical response in topline results from a Phase IIb/III trial in primary biliary cholangitis, but subtle differences in definitions, data on pruritus or itching and other factors could be key to approval and adoption.
Norucholic acid and leriglitazone, for treating primary sclerosing cholangitis and cerebral adrenoleukodystrophy, respectively, are among 12 new drugs that the European Medicines Agency has started to review for potential EU marketing approval.
The European Medicines Agency has started reviewing for potential EU marketing approval six new products, including MaaT Pharma’s microbiota therapeutic for acute-graft-versus-host disease, and AstraZeneca's camizestrant for locally advanced or metastatic breast cancer.
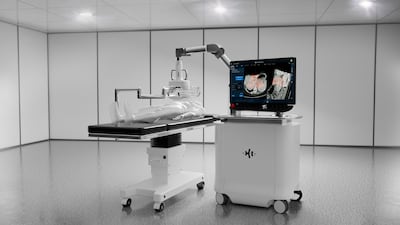
HistoSonics, which developed a noninvasive technology to destroy tumor cells, reports 90% local tumor control at 12-month follow-up in the #HOPE4Liver Trial. The Edison System, cleared by the US FDA in late 2023, is also being evaluated for kidney and pancreatic tumors. CEO Mike Blue said the medtech is financially secure but watching public markets as it considers an IPO.
Novo Nordisk’s application seeking EU marketing approval to use its GLP-1 receptor agonist to treat cirrhotic metabolic dysfunction-associated steatohepatitis is one of 15 new drug filings the European Medicines Agency has started reviewing. Meanwhile, Arrowhead’s filing for its familial chylomicronemia syndrome, plozasiran, is being fast tracked.
Ochre Bio co-founder and CSO Quin Wills spoke with In Vivo about the UK-based company's novel approach to finding RNA therapies for chronic liver disease.
From a $7m discarded asset to a $336m commercial portfolio, In Vivo looks inside Mirum Pharma’s disciplined strategy for identifying, acquiring and revitalizing overlooked rare disease programs while building toward billion-dollar potential.
An artificial intelligence-based pathology tool for metabolic dysfunction-associated steatohepatitis shows promise for a drug development landscape that is said to be “fraught with trials that have shown borderline results or outright failures based on liver histology.”