Neurology
The multiple sclerosis portfolio will drag revenue down more than Biogen’s growth products will boost 2026 sales, but Phase III results in lupus and kidney disease could bring two new launches.
Teva is lining up Europe as the next regulatory market for its long-acting injectable olanzapine, following its US filing late last year, as the company looks to build a global schizophrenia franchise.
The company said the NEAT trial in ataxia telangiectasia did not meet the primary and key secondary endpoints.
Biogen’s head of development reflects on transforming company strategy, surviving the Aduhelm crisis and building sustainable growth through disciplined portfolio management.
Management says it is prepared to sacrifice early volume if pricing fails to reflect the drug’s role in moderate to severe schizophrenia.
Management says it is prepared to sacrifice early volume if pricing fails to reflect the drug’s role in moderate to severe schizophrenia.
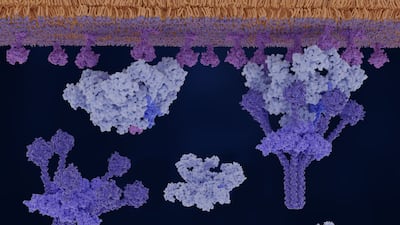
Alexion is leveraging long standing C5 leadership to expand into proximal complement targets and alternative pathway drugs, pairing longer acting and subcutaneous options with genetically guided precision medicines.
The Korean biopharma industry in 2025 was marked by some major deals involving smaller companies, while the improving investment climate could see further activity in selected areas in 2026.
Despite recent setbacks in the field, big pharma still appears to have faith in the tau approach to Alzheimer's, as evidenced by Sanofi's new $1bn-plus deal with Korean venture ADEL for an early stage candidate.
The US FDA approved Uplizna (inebilizumab) for generalized myasthenia gravis, an increasingly crowded market. Amgen believes it can compete due to the CD19-targeting antibody’s durable efficacy with twice-yearly dosing.
Teva sees olanzapine LAI as key to building a schizophrenia franchise worth up to $2bn annually, following its earlier risperidone launch.
Dyne’s data from a larger Phase I/II trial cohort showed much greater dystrophin production with z-rostudirsen (DYNE-251) than seen with Sarepta’s Exondys 51 and some functional gains.