OTC Devices
FDA updates pair of guidance documents relaxing its posture on how it regulates general wellness devices and clinical support software. Industry mostly welcomes changes, agreeing with agency’s view that lighter regulation will help spur innovation.
FDA Commissioner Marty Makary announced new guidance documents on wellness products and decision support tools focused on AI technology at CES in Las Vegas. The documents aim to reduce regulatory hurdles and promote innovation while ensuring public safety.
P&G veteran earns CHPA’s Career Achievement award; AHPA launches McGuffin Endowment Fund; Buffalo Bills QB Allen advises, promotes Therabody; tennis star serves Wild “Champions” campaign; Whoop gets entertainer’s endorsement, investment; and Tobin Scientific fills quality/regulatory SVP post.
The US International Trade Commission has banned smart rings from Ultrahuman and RingConn after Oura Health's patent infringement case. Ultrahuman countersued, claiming Oura infringes its intellectual property. Sales and marketing of the affected rings will cease on October 22.
AHPA CEO Rigby speaks at SoCal Consortium; CRN announces keynote speakers for St. Louis conference; FDA consumer health office chiefs on CHPA’s RS&Q agenda; NPA has VP For communications, regulatory affairs; COO appointed for Armorgenix; and Viatris consolidates digital, cultural, structural transfo
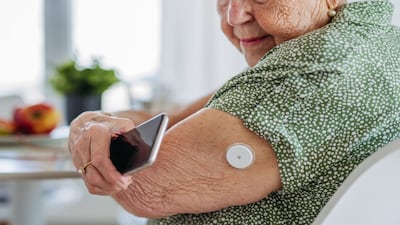
Signos links users to Dexcom’s Stelo for glucose readings and historic trends. The app is available in iOS and Android from the firm’s website, where it’s priced at $139 for three-month plan and $129 for a two-month plan, or online app stores.
Signos links users to Dexcom’s Stelo for glucose readings and historic trends. The app is available in iOS and Android from the firm’s website, where it’s priced at $139 for three-month plan and $129 for a two-month plan, or online app stores.
Reviewers were not targeted by the FDA reduction in force, but increasing attrition and difficulties hiring new staff still do not seem to have impacted new and generic drug approval totals.
Recent FDA warning letter claims Boston firm specializing in wearable technology marketed a blood pressure device without agency approval, but the company rejects the assertion and says the agency is out of step with federal regulations.
“RRAs are valuable oversight tools and under certain circumstances, can assist FDA in its mission to protect public health, oversee regulated industry, and help ensure regulated products comply with FDA requirements,” according to final guidance.
After National Advertising Division attorneys, in a review prompted by a challenge by Band-Aid line marketer Kenvue, recommended ASO LLV cease use of its “up to 2x faster healing” claim, they determined the firm had not fully complied.
CHPA Health In Hand Foundation accepting nominations for 2025 US Self-Care Marketing Awards; Celsius hires former PepsiCo as president/COO and closes $1.8bn acquisition of energy drink competitor Alani; and AdvoCare adds John Peirano as integrated marketing VP after hiring Kathy Blosser supply chain VP.