Patient Monitoring
The US FDA has published final guidance to provide clarification for industry and agency staff on federal regulations of diagnostic X-ray equipment.
Altoida CEO Marc Jones spoke with Medtech Insight about the company’s investigational digital screening tool for Alzheimer’s and the dire need for better, more accessible precision neurology diagnostics as the global population ages, neurologist shortages worsen, and groundbreaking Alzheimer’s drugs change the treatment paradigm.
The US FDA is providing recommendations for sponsors conducting clinical trials outside traditional settings, such as individual homes, mobile research units, and remotely via telehealth participation. The agency says the guidance is part of an overall effort to advance how trials are designed and run.
Edwards Lifesciences’ Critical Care business “invented the hemodynamic monitoring category, and its solutions are currently used in more than 10,000 hospitals globally to better understand the cardiovascular condition in real-time for critically ill patients, which helps improve outcomes,” says BD, which sees synergies and new innovation opportunities across the groups’ data sets and platforms. Acquired for $4.2bn cash, Critical Care generated more than $900m in revenue in 2023.
Sonio says its acquisition by global medical equipment company Samsung Medison is a wrap. Plans for the deal were announced earlier this year.
Elevated levels of the COVID-19 virus in wastewater samples have prompted the Biden-Harris administration to dust off its free testing program in preparation for the fall and winter. The US Postal Service will begin shipping the testing kits in September.
Inari Medical is updating use instructions for a clot-removing catheter due to the potential for serious adverse effects, including death.
The US FDA has granted marketing authorization to NOWDiagnostics for the first at-home over-the-counter test to detect syphilis, which the government says is on the rise.
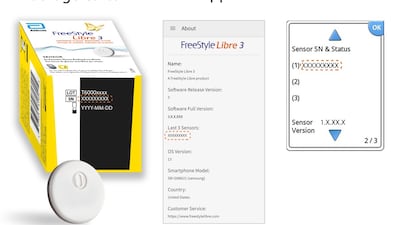
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.
The FDA has published final guidance to assist developers of medical devices designed to treat opioid use disorder, along with considerations for sponsors of clinical studies to evaluate those devices.
The US Federal Trade Commission wants to clean up the FDA’s Orange Book by purging medical device patents that the commission says should not be in the listing. The FTC argues improper patents in the Orange Book block lower-cost generic equivalents from coming to market. Medtech Insight spoke to attorney Sara Koblitz about the FTC’s delisting push.
The US FDA is reporting dozens more additional deaths associated with a May recall of Philips ventilators than initially reported. The company says it stands by its original number of seven and has reached out to the FDA.