Poisoning
The merging of the capabilities of Belgium’s SERB and the UK’s BTG Specialty Pharma could produce the world’s biggest antidotes manufacturer.
Manufacturing and supply chain resilience ensured that BTG Specialty Pharma met its sales target in the COVID-affected 2020. Its president, Anthony Higham, explained how this was possible while the company busily ̶ and quietly – sought new ownership, and all under the shadow of Brexit.
The sale of BTG Specialty Pharmaceuticals to two affiliates of European specialty firm SERB leaves Boston Scientific with BTG’s interventional oncology and vascular products.
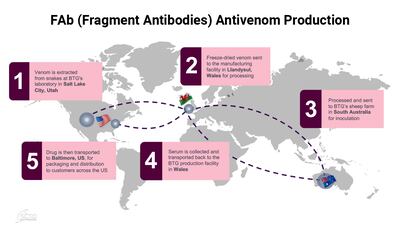
Two major initiatives have been announced which hope to revolutionize the treatment of snakebites, which claims the lives of up to 138,000 people a year.
Harm Reduction Therapeutics, which has sought funding for a US Rx-to-OTC new drug application, receives grant from Purdue Pharma that enables filing and launching the product in a targeted 100,000 retail locations within two years.
Harm Reduction Therapeutics, which has sought funding for a US Rx-to-OTC new drug application, receives grant from Purdue Pharma that enables filing and launching the product in a targeted 100,000 retail locations within two years.
FDA and FTC send joint warning letters to 11 US firms about 12 products marketed as unapproved drugs with claims about aiding in the treatment of opioid addiction and withdrawal. FDA also thanks CSPI for suggesting the agencies investigate a scourge of fraudulent drug-abuse withdrawal claims.
Addex Therapeutics says its licensing deal with Indivior to develop and commercialize its investigative addiction therapy ADX71441 'buys time' to find resources for progressing its own pipeline.
Final US guidance adds CHPA's suggestion for an example for a warning on labeling for products containing both acetaminophen and aspirin, needed to highlight where “Allergy Alert” and “Liver Warning” statements are on those products' labels.
Final US guidance adds CHPA's suggestion for an example for a warning on labeling for products containing both acetaminophen and aspirin, needed to highlight where “Allergy Alert” and “Liver Warning” statements are on those products' labels.
Much of the attention at the 2015 World Health Assembly (WHA) in Geneva, Switzerland later this month is expected to be focused on reforming the World Health Organization (WHO) and the failures by the international public health agency in responding to the ongoing Ebola epidemic in West Africa.
The FDA has given its blessing to Emergent BioSolutions' intravenous immune globulin drug Anthrasil as a treatment in combination with appropriate antibacterials for inhalational anthrax, which is caused by breathing in the spores of the bacterium Bacillus anthracis.