Prescription To OTC Switch
In contributed commentary, Susan Levy, SBL Consulting Group founder and principal, discusses likely health and wellness market developments which would reflect a steady shift toward more accessible, consumer-centric models of care complementing traditional healthcare.
In PDUFA reauthorization subgroup meetings, CHPA suggests expanding FDA’s special protocol assessment program to include OTC drug studies before FDA notes Congress has required it to provide switch-specific guidance before current PDUFA expires.
CDER Office of Generic Drugs publishes MaPP for prescription-to-nonprescription switches and ANDAs to explain regulatory responsibilities for makers of generic copies of reference listed drugs approved for OTC switch.
CDER Office of Generic Drugs publishes MaPP for prescription-to-nonprescription switches and ANDAs to explain regulatory responsibilities for makers of generic copies of reference listed drugs approved for OTC switch.
The tilt toward approval extended beyond patients and advocates to clinicians, professional societies and industry.
FDA says Teresa Michele, in CDER nonprescription drugs program leadership roles since 2013, was being moved to another position in the agency.
Agency intends to use information from comments to guide its planning for a public meeting during 2026, according to a Federal Register notice. A GAO report is due in a year to Congress profiling FDA’s progress on making more drugs available nonprescription through OTC switch applications.
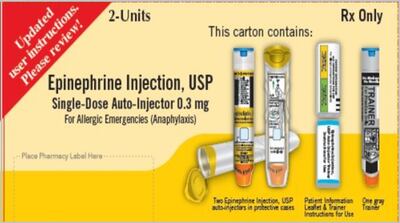
Approval of numerous Rx formulations hasn’t translated to wider availability when a remedy is needed for anaphylaxis emergency, say FDA and the Margolis Institute in announcing workshop on reducing anaphylaxis-related morbidity and mortality.
OMUFA reauthorization and directions for FDA to provide reports on monograph program and guidance on improving chances for OTC switch applications included in recent stopgap spending bill to end federal government shutdown.
Senate “SMART OTC” Act would require the FDA to identify product categories that could be eligible for an Rx-to-OTC switch and create a framework to work with sponsors.
Amphastar confident Primate Mist asthma spray will lose little market share to eventual generic competitors because it’s developing a propellant with a lower ozone-depleting impact. Also looks for US OTC market revenues from proposed naloxone nasal spray.
Results of survey of 986 oral contraceptive users 15 to 45 years old published online in JAMA Network Open show OTC access drove a 31.8 percentage-point increase in women shifting from no contraceptive method to an effective method.