Rare Diseases
The company announced positive Phase II results for the JAK1/TYK2 inhibitor in cutaneous sarcoidosis, which has no approved treatments.
The European Medicines Agency is set to adopt opinions on whether marketing approval should be granted to five new products, including therapies for a serious liver disease and an ultra‑rare genetic mitochondrial disorder.
ANI’s Cortrophin Gel is benefiting from significant under-penetration in areas such as nephrology, neurology, rheumatology and pulmonology, the firm told attendees to the recent J.P. Morgan Healthcare Conference.
Biogen’s head of development reflects on transforming company strategy, surviving the Aduhelm crisis and building sustainable growth through disciplined portfolio management.
The company plans to take Aqneursa to regulators in the US, Europe and elsewhere for ataxia-telangiectasia (A-T), which currently lacks any approved therapy.
Zydus is to gain from a world-first nod for a Menkes disease drug in the US amid speculation on a deal to acquire Ardelyx. Scrip examines how Zycubo approval, Agenus’s oncology BOT/BAL combination progress and a Formycon partnership help the Indian major
The Niemann-Pick disease type C drug is off to a good start in the US but Europe may be even more lucrative, CEO Neil McFarlane tells attendees at the J.P. Morgan meeting.
Amphastar is looking to a “strategically important addition to our growing proprietary peptide portfolio,” after striking an exclusive licensing agreement with China-based Nanjing Hanxin Pharmaceutical Technology.
A deal for the Duchenne muscular dystrophy treatment with Nxera is valued at up to $205m.
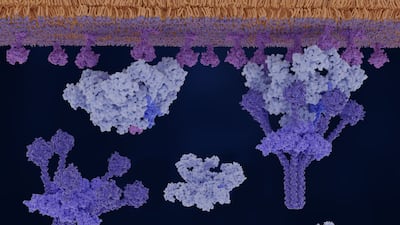
Alexion is leveraging long standing C5 leadership to expand into proximal complement targets and alternative pathway drugs, pairing longer acting and subcutaneous options with genetically guided precision medicines.
Products likely to be subject to evidence generation agreements under England’s Innovative Medicines Fund are those that are costly and which serve a low number of patients. Orphan drugs appear most likely to receive interim funding through the IMF.
The drugmaker announced that the pivotal trial of fidrisertib in FOP did not meet its primary endpoint, but it already markets the first ever drug to win FDA approval for the disease.