Recalls
When FDA warned Agebox about selling iKids-Growth IGF-1 Support supplements as unapproved drugs, agency along with CDC had for around a month been investigating outbreak of infant botulism linked to ByHeart formula.
MediNatura expands recall to include all lots of both ReBoost and ClearLife nasal sprays after microbial contamination and yeast or mold were found. Firm in 2021 challenged homeopathic drug oversight change from policy in place since 1988.
The recall of various Respironics sleep and respiratory care devices in 2021 plunged Philips into static growth and market share loss. Careful remediation is the group’s top priority, but S&RC head Dan Leonard is determined that the manner of its achievement will be the envy of the industry.
The recall of various Respironics sleep and respiratory care devices in 2021 plunged Philips into static growth and market share loss. Careful remediation is the group’s top priority, but S&RC head Dan Leonard is determined that the manner of its achievement will be the envy of the industry.
Brent Wisner suggests FDA “should be focusing on slowing down and making sure they get it right. Because at the end of the day, getting it right is more important than making sure drug companies can make more money. That shouldn't be the priority.”
“Company employee diverted legitimate packaging and customer information to distribute counterfeit, adulterated product,” says Green Lumber. It’s “working cooperatively with FDA and law enforcement to address this matter.”
PCH lowers full-year guidance even after reaching $100m deal to acquire Canadian sterile ophthalmic manufacturer for its Clears Eyes line troubled by supply slumps for several years. Its $47.5m reported net sales for April-June period were down 6% from a year ago.
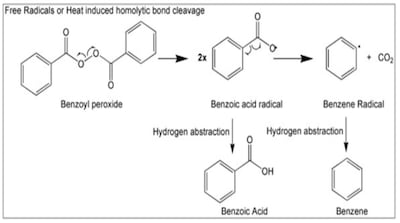
FDA testing of 95 benzoyl peroxide products due to concerns about elevated benzene detected by third-party testers found 90% with undetectable or extremely low benzene levels.
Kyle Diamantas was a partner with the Jones Day firm when he was tabbed as acting deputy commissioner to lead the FDA’s Human Foods Program, established in the agency’s reorganization which became effective in October.
The Therapeutic Goods Administration is advising companies to get up to speed with its new recall procedure, which is designed to improve the timeliness of recalls, alerts and corrections, and reduce regulatory burden for sponsors.
The Therapeutic Goods Administration is advising companies to get up to speed with its new recall procedure, which is designed to improve the timeliness of recalls, alerts and corrections, and reduce regulatory burden for sponsors.
Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.