South Africa
The disposal of the Asia-Pacific business outside China to BGH Capital allows Aspen to refocus on core growth drivers, including GLP-1 products and restoring profitability in sterile manufacturing.
A new reliance pathway under which regulators will rely on each other’s assessment reports strengthens the goals of key continental health initiatives, including the newly formed African Medicines Agency.
Natco Pharma has inched closer to completing its planned INR20bn acquisition of a 35.75% stake in South Africa’s Adcock Ingram, after the latter’s shareholders overwhelmingly approved a scheme of arrangement, with 98.66% votes in favor.
Adcock Ingram celebrated a minor turnaround in the back half of its 2025 financial year, though its Prescription division trading profit still fell well short of last year’s level.
Aspen Pharmacare was hit hard by a contractual dispute in its 2025 financial year. But the firm has disclosed a strategy to recoup lost earnings, while pushing the strength of its API division.
In the Delhi High Court, Natco Pharma has initiated proceedings against Novo Nordisk, requesting a ruling of non-infringement regarding a patent tied to semaglutide, the ingredient in the Danish originator’s Ozempic and Wegovy brands.
Natco to buy out Adcock Ingram’s minority shareholders in what’s possibly its biggest M&A investment, opening up substantial opportunities in South Africa and the region. If the offer goes through, it will result in the delisting of Adcock, with Bidvest and Natco co-owning the firm.
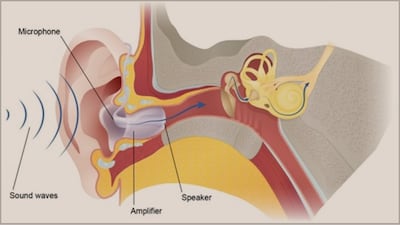
The firms recently announced they will operate as combined company LXE Hearing marketing Eargo’s namesake line and hearX’s Lexie brands. Eargo majority owner Patient Square Capital added $100m to its investment.
The firms recently announced they will operate as combined company LXE Hearing marketing Eargo’s namesake line and hearX’s Lexie brands. Eargo majority owner Patient Square Capital added $100m to its investment.
Cipla has bolstered its ophthalmology portfolio and increased its global offering after chalking up an agreement to add Formosa Pharmaceuticals’ USFDA-approved clobetasol propionate 0.05% ophthalmic suspension.
South Africa’s Aspen Pharma has discussed the “significant progress” it believes it has made in GLP-1s, with an eye on rolling-out semaglutide in non-US and non-EU markets beginning as early as 2026.
South Africa’s Adcock Ingram struck a downbeat tone during its financial first-half results call, as multiple factors conspired to produce a performance “well below expectations.”