Tracking Trials
The first reported analysis demonstrating a patient-level dose response with Novocure’s Tumor Treating Fields therapy is now published. The post-hoc analysis of the EF-14 trial showed higher doses of Tumor Treating Fields improved survival in newly diagnosed glioblastoma patients.
Sensus announced top-line results from a five-year restrospective study of its SRT-100 Superficial Radiation Therapy device for non-melanoma skin cancer. SRT-100 received a 510(k) in 2018.
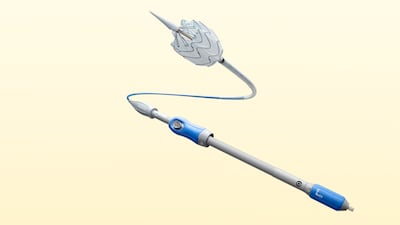
SurModics suspended the trial with their SurVeil drug-coated balloon in March after the US FDA sent a letter to physicians alerting them to possible safety problem with paclitaxel-coated devices. The company has updated the patient consent form and established a new patient follow-up program for the trial.
The study will be expanded by 50 patients, for a total of 220 subjects, based on the recommendation of a statistician who reviewed an interim analysis. RECON is intended to support a Biologic License Application for Avance, a processed human nerve allograft for bridging severed peripheral nerves.
The Woven EndoBridge (WEB) aneurysm embolization system proved relatively safe and effective in the 150-patient WEB-IT trial. The trial results are now published in the Journal of Neurointerventional Surgery.
Medtronic is encouraged by new data on endovascular repair of abdominal aortic aneurysm presented at the Charing Cross Symposium, including preliminary data from the ANCHOR registry on Endurant used in combination with the Heli-FX Endoanchor system. At the same meeting, 30-day results from 100 patients in the pivotal trial of Valiant Navion thoracic stent graft system showed high rates of procedural success in patients with a thoracic aortic aneurysm and penetrating atherosclerotic ulcer.
New trial results show Guardant Health's Guardant360 largest cell-free DNA assay rapidly identifies biomarkers for metastatic non-small cell lung cancer associated with specific therapies at least as accurately as standard-of-care tissue genotyping.
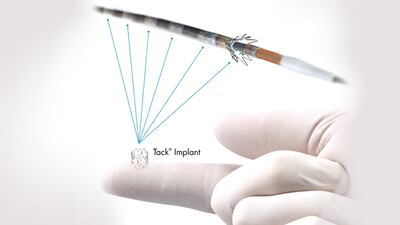
The approval of the Intact's peripheral artery dissection repair device is based on results from the 213-patient TOBA II trial, which showed Tack resolved 92.1% of dissections in peripheral arteries following balloon angioplasty.
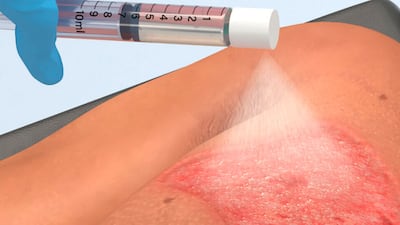
The Recell system of Spray-On Skin Cells combined with mesh autografts healed 98% of burns within four weeks in 23 pediatric patients treated under US FDA-approved compassionate use and continued access programs.
SPR Therapeutics' Sprint percutaneous peripheral nerve stimulator proved safe and effective at reducing pain in post-amputation patients in a new 28-patient randomized study.
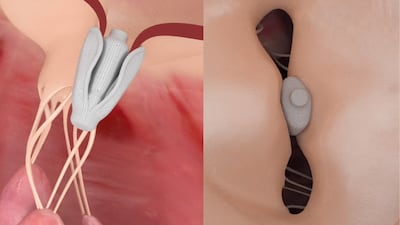
One-month results from the CLASP trial, presented at the German Cardiology Meeting (DGK), show Edwards’ Pascal mitral valve repair device reduces mitral regurgitation in heart failure patients with moderate to severe mitral regurgitation.
A post-hoc analysis of the combined data from the ENDURANCE and ENDURANCE Supplemental trials shows a relationship between severely disabling stroke and one-year mortality in patients treated with Medtronic’s HeartWare HVAD as well as other left-ventricular assist devices (LVAD), including Abbott’s HeartMate II, and that blood-pressure control significantly reduces the risk of stroke events from six-months to two-years post-implant.