Drug Pricing
After taking the reins as president of Medicines for Europe at the start of 2026, Zentiva CEO Steffen Saltofte sets out priorities for the group that include ensuring access to medicines, industry resilience and competitiveness, harmonized regulation, and sustainability for the off-patent sector.
With another settlement secured, US states are gearing up for the first trial of the price-fixing case later this year.
The health technology assessment institute said its recommendation for the multiple sclerosis drugs, Tysabri and Tyruko, highlighted its continued efforts to drive the adoption of biosimilars across the National Health Service.
As more medicines come off patent in the coming years, the UK generics and biosimilars industry association is hoping to see more generic products evaluated for reimbursement by the health technology assessment institute, NICE.
Sandoz’s CEO used the firm’s latest results call to highlight “trade distortion” in the pencillins market as a result of US tariffs, urging European authorities to take action to reduce the region’s “geopolitical exposure” and safeguard the long-term sustainability of European-produced penicillins.
Companies have been given two extra weeks to decide whether they want to leave the UK’s voluntary pricing scheme for branded medicines, adding to a previous four-week deadline extension.
The AAM has asked for a federal injunction to block a Connecticut state law on drug pricing that it says represents “an unconstitutional overreach that threatens patient access to affordable medicines.”
Through an expansion of their partnership, Biocon Biologics and Civica Rx are planning to launch a private-label version of insulin glargine in the US from January – at a price that equates to just $11 per pen.
While branded generics and biosimilars remain subject to the UK’s VPAG pricing scheme, Sandoz’s public affairs lead has argued that focusing on driving competition and uptake rather than rebates is a better way to generate savings – and that off-patent products should be exempt from VPAG altogether.
The AAM and Biosimilars Forum have criticized a rule adopted by Colorado’s Prescription Drug Affordability Board to establish an upper payment limit for Enbrel – well ahead of market entry for etanercept biosimilars.
The European generics industry has welcomed news that generic medicines will be exempt from tariffs under the latest EU-US trade agreement.
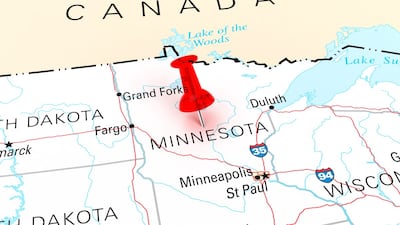
The AAM has welcomed a US appeals court decision backing an injunction against a Minnesota state law on drug pricing, following an earlier favorable district court ruling.
Sun readies US debut of Leqselvi for alopecia areata, while PD-L1 inhibitor Unloxcyt is in the line up once the Checkpoint Therapeutics deal is completed. Management also discusses limited clarity on President Trump’s most favored nation pricing push, pipeline tweaks and M&A outlook.
Donald Trump’s executive order on most-favored-nation prescription drug pricing could provide positive opportunities for the off-patent industry, although US generics and biosimilars association the AAM suggested that the administration might do more good by focusing elsewhere.
After the product’s initial launch in May 2024, California’s government has now expanded the availability of its CalRx-branded generic OTC Narcan to all of its citizens.
Optum Rx, one of the largest PBMs in the US, declared itself as the “first comprehensive, transparent pharmacy services company” after announcing several consumer-friendly changes.
Walmart has launched an attack on a host of industry-leading generics firms over historical price-fixing allegations, with the US retail giant seeking treble damages from a Pennsylvania district court.
In the wake of Formycon’s recent announcement that US marketing partner Sandoz would be pausing commercialization of the Cimerli rival to Lucentis that it recently took over from Coherus, Sandoz management has offered a few more details on its plans for the ranibizumab biosimilar.
Sandoz CEO Richard Saynor has spoken of his frustration at a lawsuit from J&J seeking to prevent the launch of a private-label ustekinumab biosimilar in the US, which he described as “the desperate actions of an originator trying to protect its market.”
Discussing a new report highlighting the lack of US biosimilar competition on the cards for the majority of biologics losing exclusivity in the next ten years, the AAM’s Access! 2025 conference heard that the “sobering” findings should act as a “wake-up call for stakeholders.”