Innovation & IP
AI advisers could eventually guide consumers toward health products based on personalized needs rather than traditional marketing, IQVIA Consumer Health suggests. But brands will still need to balance data‑driven recommendations with the human appeal of in‑store discovery and self‑care experiences.
Germany’s Expert Committee for Prescription (SVA) has unanimously rejected the proposed switch of the controversial painkiller metamizole from prescription to OTC status.
Futura Medical "delighted" with early feasibility study results for topical OTC treatment WSD4000.
Germany’s Expert Committee for Prescription meets on 20 January to discuss the Rx-to-OTC switch of controversial painkiller metamizole, which has been banned in a number of countries around the world because of the risk of agranulocytosis.
AI is rapidly reshaping consumer health advertising across Europe, supporting creative work, compliance checks and regulatory oversight, according to a recently published report by UK consumer healthcare industry association, PAGB.
Consumers are increasingly seeking health and wellness solutions that combine natural ingredients with scientific credibility and technological innovation, reflecting a shift from treating illness to embracing prevention, according to the latest trends report from UK retailer Holland & Barrett.
When conducted according to a rigorous protocol, incorporating validated questionnaires, and meeting the standards for peer-reviewed publication, real world surveys deliver enhanced value, says IQVIA Consumer Health.
With the grant of a patent, Futura is hoping to take its Eroxon product to China, a country with a large population of ED sufferers, the firm claims.
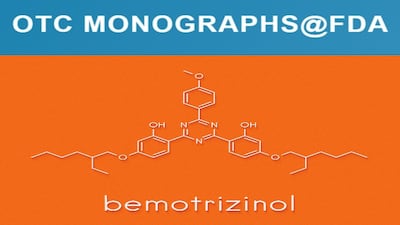
FDA’s proposed order follows its review of OMOR DSM-Firmenich submitted showing bemotrizinol, at concentrations up to 6%, is generally recognized as safe and effective and can be added as an active ingredient to sunscreen monograph.
“In the coming years, growth will depend on whether brands can combine trust, personalization, and proof of evidence into one coherent prevention experience,” argue Simon Kucher's Clemens Oberhammer and Christian Rebholz in this exclusive interview.
The trophies have been presented at the OTC Marketing Awards 2025 - but what did the judging panel have to say about the winning and highly-commended entries?
Winners across 12 categories were announced at the OTC Marketing Awards 2025 on 20 November, with Opella crowned OTC Company of the Year.
Self-Care Week Special: HBW Insight catches up with PAGB CEO Michelle Riddalls OBE to talk about what the association is doing to promote Rx-to-OTC switch in the UK.
“It’s not good enough just to provide products anymore, we need to create a service and a holistic approach to products,” argued Bayer Consumer Health's Northern Europe general manager, Mike Knowland, speaking at a recent panel event in the UK Parliament.
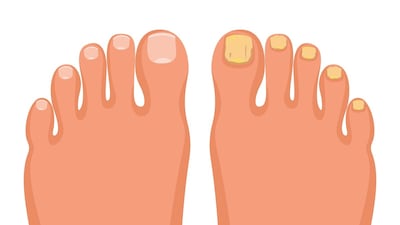
Karo will take on responsibility for the marketing, distribution and sales of Moberg’s topical onychomycosis treatment, MOB-15, under the Lamisil brand in 19 European countries.
The global allergy market is “expanding again but on new terms, driven by a mix of regional strength, evolving consumer behaviors, and an increasingly diverse competitive field,” according to the latest IQVIA Consumer Health analysis.
Japan’s Ministry of Health, Labor and Welfare continues to widen access to non-prescription medicines with another Rx-to-OTC switch – Rohto Algard Epinastine Eye Drops – and a direct OTC approval of Sato Pharmaceutical's Menofeminin (black cohosh) menopause treatment.
P&G expands its WICK range with a new natural line of cough syrups; Angelini introduces a guideline-compliant Tantum Verde Duo throat spray; Zentiva adds a children’s fever product to its Ibuflam brand.
In overall terms, yes, answers IQVIA's latest Digital Health Trends report. But the market for condition-specific apps, particularly if they combine this with a high-quality user experience and strong clinical evidence, is booming.
The shortlist has been revealed for the upcoming 30th Annual OTC Marketing Awards, which takes place on Thursday 20 November in London, UK.