FDLI
Limited visibility into the FDA’s use of remote regulatory assessments and the lack of a clearly defined closeout process leave companies uncertain about outcomes and expectations, industry experts say.
Former agency officials who now represent industry worry that a deregulatory bent could be driving the "Simple Reform" plan to merge all medical product and clinical research inspectorates and that the specialist expertise gained in the 2017 "Program Alignment" initiative will be reversed.
The October 2024 reorganization that moved compliance functions back into the product centers has resulted in speedier issuance of biologics warning letters and an increase in drug manufacturing facility classifications, FDA compliance officials said.
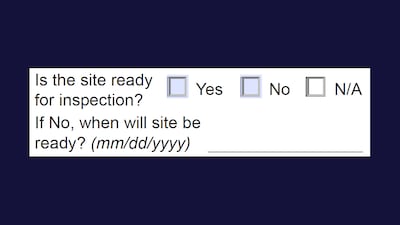
Facilities that are not ready for an inspection can lead to delays in the FDA’s site assessment and leave little time to resolve deficiencies ahead of a user fee goal, CBER compliance office Director Melissa Mendoza said.
The first round of ad/promo violation letters largely left social media untouched despite a vow to go after “dark ads” and violative influencer claims. Industry wants more information on the new, de facto standards suggested in the initial DTC and healthcare provider promotion letters.
Sponsors should consider whether their TV commercials contain the same number of scene changes during the major statement as ads that received a violation letter.
The extent of FDA and Justice Department coordination on recent warning and untitled letters is unclear, but the “squishy” issues cited in many of the letters and recent elimination of DoJ’s Consumer Protection Branch could influence legal action, industry lawyers said.
An AI tool developed by the Office of Prescription Drug Promotion for reviews is believed to still lack generative capabilities, said former senior staffer Jason Cober, who also raised concerns about intellectual property protections for sponsors’ advertising and promotion submissions.
Robert Foster, HHS deputy general counsel and chief counsel for food, research and drugs, is temporarily heading the Office of Chief Counsel, but a permanent appointee is expected after Michael Stuart's confirmation as HHS general counsel.
A 26 June deadline looms for the final guidance even though a draft guidance was removed from the agency’s website to comply with President Trump’s gender ideology executive order. The concepts underpinning the guidance are still relevant, legal experts say.
Bridget Dooling, a law school professor who reviewed draft regulations from the FDA and other agencies as an OMB attorney, said prior federal court decisions suggest judges typically defer to agency decisions based in science.
Deputy FDA Commissioner Grace Graham acknowledged the importance of user fees, while also calling for restructuring. She also said part of MAHA's mission is to reexamine uses of drugs not supported by data.
New CBER Director Prasad will "unleash a massive" framework on vaccines, FDA Commissioner Marty Makary said 15 May, raising questions about the impact on products nearing approval.
The agency’s compliance directors also discussed expectations for greater inspection and review efficiencies as part of the 1 October reorganization during a recent FDLI conference.
The compounding industry ties for Martin Makary, President-elect Trump’s candidate to lead the FDA, could mean less compounding enforcement, experts said, but government officials said their enforcement focus will remain nonpartisan.
The loss of institutional knowledge about OPDP’s prior comments on promotional materials can result in an enforcement letter for the new owner of a company or product, experts say.
Written contracts should be detailed, require compliance with FTC and FDA requirements, and guard against activities that may damage a company’s reputation, experts said.
Advertisers are facing a 20 November deadline to bring TV and radio ads into compliance, but stakeholders still question the reg’s scope, including whether and how it applies to ads on streaming services and social media platforms. FDA advisory comments suggest the agency is taking a hard stand on the rule’s ‘dual modality’ requirement.
Multiple and repeated complaints will sharpen the Office of Prescription Drug Promotion’s focus on an advertisement, Director Catherine Gray said, while Foley Hoag partner August Horvath said the self-regulatory NAD process is best suited to complaints that lack a ‘great scientific basis’ for objecting.
Firms can be more forceful in disputes with FDA now that Supreme Court has eliminated Chevron deference. Questions around whether an evidentiary standard has been met may be ripe for challenge, legal experts said, but they also caution that sponsors will face more uncertainty.