AAM
After 10 years of adding suffixes to all new biologic and biosimilar nonproprietary names, FDA officials are considering whether it is still necessary for pharmacovigilance purposes.
Elevating the Office of Therapeutic Biologics and Biosimilars out of the Office of New Drugs and providing signatory authority could help speed biosimilar reviews, OTBB Director Sarah Yim said.
Pink Sheet editors discuss FDA announcements that clinical efficacy studies would no longer be required for biosimilar development, as well as the additional information from the agency on the intent of its “Simple Reform” of the inspection staff.
The new guidance says comparative clinical trials are not a prerequisite for all biosimilars, a notable step for the FDA, but the commissioner added that biosimilar prices should be significantly lower than the reference product at launch.
BIO
Pink Sheet and Scrip journalists reflect on the mood from Boston, the most important takeaways and what’s next for industry.
John Crowley discussed how he prioritizes industry’s many competing challenges in a Pink Sheet interview at the BIO International Convention.
Highlights from Day Four of the BIO International Convention include policy concerns helping constrain dealmaking, Novartis discussing its approach to partnering, and Generate looking for funding to move into Phase III.
The former CDER director said she tells sponsors not to conduct an FDA-recommended study design or randomized trial if it will not work.
DIA
AI is moving from blue-sky potential to specific applications in drug development, streamlining pharmacovigilance and clinical trial design, but strong governance remains essential to ensure transparency and reliability.
Real-world evidence is becoming increasingly sophisticated, but fundamental issues like data reliability remain central to the discussion.
The FDA will take a hard line on trial design and site selection to ensure applicability to the US, Oncology Center of Excellence Director Richard Pazdur said during a meeting on GSK's Blenrep.
Companies can propose specific issues for discussion during the regulators’ monthly teleconferences, which focus on finding areas of agreement and reasons for nonalignment on specific pediatric development plans or general issues.
Duke-Margolis Center for Health Policy
The clarity on quality topics provided by the CMC Readiness Pilot (CDRP) is worth the work of preparing a comprehensive development review and multiple meetings, participants from Intellia, Bicycle Therapeutics and Bristol Myers Squibb said during a Duke Margolis meeting.
Center for Biologics Evaluation and Research Director Vinay Prasad and Commissioner Martin Makary will introduce the new pathway soon in a New England Journal of Medicine article.
Agency’s plan for advanced manufacturing seeks more harmonization, while also seeking to codify internal practices with guidance and training.
The agency’s plan for advanced manufacturing seeks more harmonization, while also seeking to codify internal practices with guidance and training.
FDLI
Limited visibility into the FDA’s use of remote regulatory assessments and the lack of a clearly defined closeout process leave companies uncertain about outcomes and expectations, industry experts say.
Former agency officials who now represent industry worry that a deregulatory bent could be driving the "Simple Reform" plan to merge all medical product and clinical research inspectorates and that the specialist expertise gained in the 2017 "Program Alignment" initiative will be reversed.
The October 2024 reorganization that moved compliance functions back into the product centers has resulted in speedier issuance of biologics warning letters and an increase in drug manufacturing facility classifications, FDA compliance officials said.
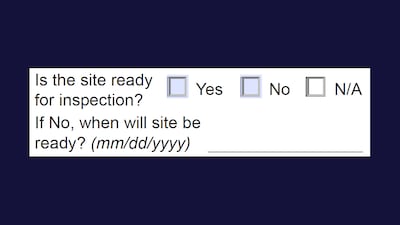
Facilities that are not ready for an inspection can lead to delays in the FDA’s site assessment and leave little time to resolve deficiencies ahead of a user fee goal, CBER compliance office Director Melissa Mendoza said.
PDA/FDA
Third-party screening of US-marketed drug products is worse than pointless if done with subpar test methods, agency tells PDA/FDA meeting. Meanwhile, release-testing with such methods has been triggering warning letters.
Third-party screening of US-marketed drug products is worse than pointless if done with subpar test methods, agency tells PDA/FDA meeting. Meanwhile, release-testing with such methods has been triggering warning letters.
Alkermes CRL is just the latest fallout from black-hole process.
Inter-associations working group suggests changes to the EU GMP Annex I revision to make it easier to understand by health authorities worldwide; the groups point out that the annex will be used globally and not just in Europe.