Elections
The Trump administration has launched TrumpRx, a website the White House says could help save Americans billions in pharmaceutical spending, although it does not itself sell or dispense drugs.
As South Korea's new president immediately gets to work on setting initial policies, essential drug supplies, R&D incentives and AI-driven digital healthcare are among the key topics in focus.
As South Korea gears up for a snap presidential election, its biopharma industry proposes key desired policies to candidates, most of whom have not laid out detailed plans of their own for the sector.
Analysts expect a limited impact on South Korean pharma from US tariffs, even if imposed at a later date. Meanwhile, the early June domestic presidential election is set to determine the direction of policies in the sector.
Biotech execs and lobbyists try to explain the Trump Administration's policy strategy and how to thrive in it.
Robert F. Kennedy Jr. will become HHS secretary while retaining his anti-vaccine positions. His first action likely will be creating a new safety commission, which industry must hope holds off more drastic action.
At a lively AAM Access! conference in Florida, AAM President and CEO John Murphy acknowledged the negative effects of potential tariffs, but also suggested that the second Trump Administration could be an opportunity for the generics and biosimilars sectors to advance market reforms.
Pink Sheet reporter and editors discuss what pushed a crucial Senate Finance Committee swing vote to support Robert F. Kennedy Jr. as HHS Secretary and the FDA slowly expanding its communications with the public amid the Trump Administration freeze.
The FDA's recent communications freeze has blocked participation in international standards development groups, which could impact the alignment of US and international requirements.
The Trump transition so far has all the hallmarks of the tech industry’s favorite management slogan, but that might not work well for biopharma companies if the FDA is one of the agencies that gets broken.
CDER and CBER once again posted net employee gains, even during the quarter that included the presidential election, despite fears that many would leave rather than endure the changes to be imposed by President Trump.
The chair of the Senate Health, Education, Labor and Pensions Committee questioned whether Kennedy could reject anti-vaccine views in the face of data affirming their safety and efficacy.
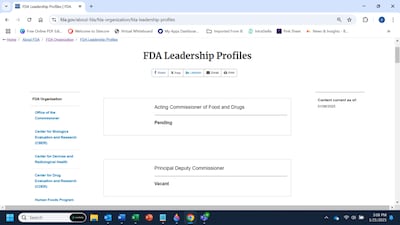
The FDA's interim leadership takes shape, but the sense of transition chaos still lingers.
Pink Sheet reporter and editors discuss the uncertainty created by the Trump Administration freezing most communications at the Health and Human Services Department, new travel and telework bans, as well as the delay in announcing an acting FDA leader and the impact of new executive orders on the FDA and CMS.
The cell and gene therapy field has reached an inflection point in the US as the second Trump Administration begins and advocates argue the sector could fit well with the “MAHA” agenda.
The White House is expected to name Sara Brenner, chief medical officer for in vitro diagnostics at CDRH and a veteran of the first Trump administration, as acting commissioner, but the lack of a formal announcement is creating uncertainty. New executive orders on telework and the senior executive service also could impact agency staff.
Pink Sheet Podcast: US FDA And Trump’s Reforms, CDER Director Parting Comments, 2024 Approval Trends
Pink Sheet editors consider ex-FDA officials’ advice for the Trump Administration on implementing FDA reforms, comments CDER Director Patrizia Cavazzoni made before her departure from the agency about wanting to release review documents for applications that received complete response letters, and diverging trends between CDER and CBER novel application approvals.
Robert Califf’s final appearance on Capitol Hill as US FDA commissioner, a 5 December Senate hearing focused on the agency’s food and nutrition functions, was an appropriate coda to his tenure and preview of things to come under the Trump Administration.
Former FDA commissioners Mark McClellan and Scott Gottlieb, former acting commissioner Janet Woodcock and current commissioner Robert Califf offered advice on successfully implementing reforms and preventing a mass exodus of FDA employees as inklings emerge that the Trump team is already engaged on this front.
6 January 2025 will be remembered in Washington, DC, as the date Congress certified Donald Trump’s second presidential election victory and a snowstorm shut down most of the city, but for FDA watchers it will be the day the agency released more than two dozen new draft and final guidances.