Manufacturing
Recommendations to mitigate attrition among clinical research and drug manufacturing investigators at the FDA have not been implemented, and a Strategic Workforce Plan aimed at addressing recruiting, retention and training challenges has been shelved, the Government Accountability Office said.
The FDA wants to waive facility fees for three years if a sponsor breaks ground on a US-based manufacturing plant, but industry is concerned the idea will force other sponsors to subsidize competition.
Sponsors now must specify domestic manufacturing facilities and bioresearch monitoring study sites at least 60 days before the complete application is submitted, which appears intended to ensure inspections keep pace with the compressed review timelines.
The European Parliament has adopted its position on the Critical Medicines Act, prompting industry representatives to highlight ongoing areas of concern in the proposal.
The Swiss pharma co-authored a “call for bold life sciences investment” with the Eurasia Group, critiquing global trade policy and advocating “innovation-friendly” reforms, including moving away from reference pricing.
Regulatory streamlining including in areas such as test licenses and cell/gene therapy, and policy support/incentives to propel innovation, were among the key themes that played out in India in 2025. More action is anticipated in this year, including in the biosimilars segment.
Changing regulatory standards for cell and gene therapies extend the flexibility with manufacturing while the tabelecleucel complete response letter showed high expectations for rigor in clinical trials.
The announced agreement means AbbVie and Regeneron are the only companies of the 17 notified by the White House in July yet to make a deal.
The UK’s latest economic budget, which will enter into force this April, offers “nothing” to support the government’s claim that it is making it easier for startups to launch, scale and remain in the UK, a biotech founder and lawyer argues.
The FDA wants to extend the goal date for some applications with facilities that receive a pOAI classification to allow more first-cycle approvals.
The UK’s roadmap for reducing animal testing is a positive starting point, but greater transparency from the drug regulator and a more detailed workplan from government will be required to make the plans a reality, an expert from Cruelty Free International says.
Limited visibility into the FDA’s use of remote regulatory assessments and the lack of a clearly defined closeout process leave companies uncertain about outcomes and expectations, industry experts say.
Martin Makary’s photo-op and videos promoting the groundbreaking of a new Novartis US-based manufacturing facility create an appearance of favoritism, President George W. Bush’s former chief ethics lawyer told the Pink Sheet.
Former agency officials who now represent industry worry that a deregulatory bent could be driving the "Simple Reform" plan to merge all medical product and clinical research inspectorates and that the specialist expertise gained in the 2017 "Program Alignment" initiative will be reversed.
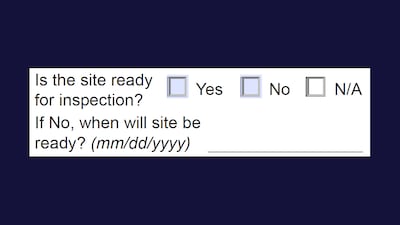
Facilities that are not ready for an inspection can lead to delays in the FDA’s site assessment and leave little time to resolve deficiencies ahead of a user fee goal, CBER compliance office Director Melissa Mendoza said.
Changes to the procurement of critical medicines and their active ingredients are among the amendments the Council of the EU has made to the draft Critical Medicines Act.
New approach methodologies are increasingly shaping the future of medicine development by making drug testing less reliant on animals.
The FDA wants to charge a yearly fee after an IND is filed for firms that do not conduct Phase I clinical studies in the US as part of its proposal to encourage more domestic development.
The UK’s autumn budget failed to introduce any widespread changes for pharma, but scale-ups and innovators could benefit from changes to enterprise and investment schemes. Some industry voices warn of underinvestment in the MHRA and innovative medicines.
The FDA included storage and handling instructions common in labeling in its approval announcement, suggesting concerns remain about the potential for NDMA to form after products are distributed.