Spain
Spain is drafting a new digital health bill that aims to ensure the country is ready to implement the European Health Data Space Regulation.
Spain has widened the pool of Phase I clinical trial applications that are eligible for faster evaluation and approval processes.
A new plan to tackle medicine shortages proposes to develop a set of incentives for companies to manufacture essential medicines in Spain.
Spanish authorities say that that timelines for drug reimbursement decisions can be improved further with the right submissions from companies.
Spanish authorities have published more pricing and reimbursement reports as part of a new drive to increase transparency.
Proposals to reform Spain’s reference pricing system are out of whack with the national pharmaceutical strategy published in December, six industry associations have warned.
The first reimbursement reports published by the Spanish health ministry to improve transparency focus on CSL’s Hemgenix, BMS’ Camzyos and Pfizer’s Velsipity.
CSL Behring explains how it worked with authorities in Denmark, Austria, England, Scotland, Spain and Switzerland to secure innovative access arrangements suitable to each nation’s “unique needs” for its one-time gene therapy, Hemgenix.
The strategy includes a committee made up of pharmaceutical industry representatives and ministers to discuss the impact of regulatory proposals.
A draft royal decree would pave the way for more transparency in pricing and reimbursement decision-making processes and boost competition to increase the participation of generic medicines.
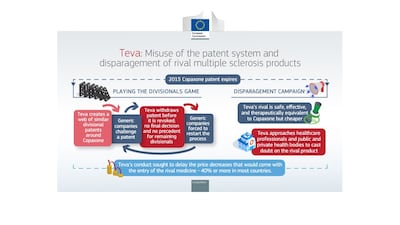
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.
Hemgenix has now secured reimbursement in several European markets, with more talks ongoing. While the path to reimbursement has not always been easy, innovative access deals have helped to smooth the way.
New rules on health technology assessments in Spain make room for real-world evidence and early dialogue.
J&J says it has “exhausted all current viable avenues” to get its antidepressant nasal spray Spravato reimbursed on England’s National Health Service, after NICE decided against re-appraising the drug following numerous funding rejections.
Santhera Pharmaceuticals did not provide enough evidence to demonstrate that its Duchenne muscular dystrophy drug Agamree was a cost-effective use of resources, according to draft guidance from England’s health technology assessment body, NICE.
Spanish competition regulator the CNMC has fined Merck Sharp and Dohme €39m for abusing its dominant position, by blocking Insud Pharma’s rival to the Nuvaring vaginal contraceptive ring through “unjustified legal action” that sought to delay market entry of Insud’s Ornibel version. The originator is considering an appeal.
France is set to become the first country in Europe to fund Imcivree for a second – and as yet unapproved – indication. Meanwhile, England has agreed to reimburse the drug for its approved indication as did Germany earlier this year. Reimbursement discussions are underway in the rest of Europe.
With efforts continuing to maximize access to medical countermeasures against the pandemic threat, a webinar organized by NGOs looked at progress with and the shortcomings of the WHO’s C-TAP initiative, recent moves by the WHO on access provisions in funding agreements, and plans for a global C-TAP database.
The current system means Spain is not a priority launch country.
EU stimulus funding marks opportunity to establish health technology appraisal agency in Spain.