Germany
The pharmaceutical industry has been pushing back on a deeply unpopular rebate on products deemed to be “free combination” medicines in Germany.
The proportion of AMNOG benefit assessments leading to a positive rating, which has a direct impact on drug pricing, has decreased in recent years, according to analysis from the VFA, the association representing the German pharmaceutical industry.
Newly enacted standard contractual clauses for negotiating clinical trial agreements in Germany appear positive for sponsors compared to the clauses that trial sites were typically requesting in the past, eg, those relating to compensation for the transfer of inventions, according to lawyers.
Health technology assessment authorities in Germany have concluded that CSL Behring’s Andembry offers significant additional benefits for hereditary angioedema patients, which could strengthen the company’s position during pricing negotiations.
IQWiG, Germany’s health technology assessment body, is making contingency plans in case key US resources it relies on for information retrieval, when conducting benefit assessments of new medicines, become unavailable.
A blanket pricing agreement CSL has formed with German health insurers for the gene therapy, Hemgenix, makes the cost of treatment budget-neutral compared to traditional treatment.
A German ordinance implementing the EU Health Technology Assessment Regulation offers little clarity on how far joint clinical assessment reports should be considered by national authorities.
Payers and health technology assessment bodies in the Netherlands, Germany and Italy are either unwilling to use real-world data in assessments or cannot due to their existing frameworks, say representatives from Gilead Sciences and Autolus Therapeutics.
An ordinance came into effect on 8 March to ensure that joint clinical assessments can be incorporated into the German pricing and reimbursement system.
Trial sponsors in Germany should start preparing negotiation strategies to tackle difficult discussions with trial sites over standardized clauses.
The world’s first CRISPR gene editing therapy, Casgevy, has been made available to patients with sickle cell disease in England, adding to access arrangements in the US, Austria, Bahrain, Germany, Luxembourg, Italy and Saudi Arabia.
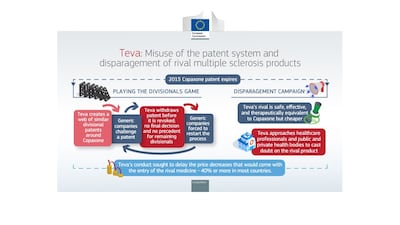
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.
Germany’s Medical Research Act has cleared its final legal hurdle after the federal council signed it off.
The revised EU Urban Wastewater Treatment Directive, which obliges pharmaceutical and cosmetic industries to contribute at least 80% towards the costs of removing micropollutants from wastewater through quaternary treatments, will place an additional burden of around €2bn per year on German manufacturers, says Pharma Deutschland.
In this third article of a series on new drug reimbursement recommendations by the HTA body NICE, the Pink Sheet finds that fewer innovative medicines are reimbursed in England than in eight other European nations.
A new act in Germany does not go far enough to address the deeply unpopular “guardrail” link between Amnog benefit assessment ratings and price negotiations, which can deter companies from launching.
J&J says it has “exhausted all current viable avenues” to get its antidepressant nasal spray Spravato reimbursed on England’s National Health Service, after NICE decided against re-appraising the drug following numerous funding rejections.
Adult immunization programs can save “billions” for society, but their value is underestimated because of challenges around measuring broader value evidence and a lack of incentives for companies to collect this data, says the Office of Health Economics.
The European Parliament has voted for a deal agreed with the council of ministers to ensure polluters pay for cleaning up urban wastewater.
Roche’s lymphoma drug Polivy could face pricing challenges in Germany as it undergoes a full benefit assessment.