Complete Response Letters
The unusual revision of Corcept’s complete response letter suggests the FDA may be writing for a different audience now that unapproved product CRLs are being made public.
The FDA starts 2026 with 55 novel agents under review, which are detailed in Pink Sheet's interactive chart.
Review times for novel agents approved in 2025 stayed remarkably steady, hugging PDUFA timelines, maintaining high approval volumes, and foreshadowing a new debate over whether ultra‑fast, politically driven reviews will result from the Commissioner's National Priority Voucher program.
Pink Sheet editors discuss Richard Pazdur’s concerns about the Commissioner’s National Priority Voucher program and the idea that the FDA could release action packages for unapproved products in addition to complete response letters.
More complete response letters were issued than approvals for novel neuroscience candidates in 2025. Orphan neurology therapies were hit the hardest.
How the agency writes CRLs ‘will need to be carefully considered’ now that they are being publicly released, Office of New Drugs Director Mary Thanh Hai said, adding that the release of action packages is a ‘much more touchy subject.’
The Food and Drug Administration’s complete response letter for tolebrutinib reveals why the multiple sclerosis drug will not reach US patients soon: a high risk of liver injury and uncertain benefit for most patients with progressive MS, details that were missing from Sanofi’s earlier communication
While the volume of applications was one of the highest in the decade, a high rate of complete response letters winnowed the pool to an approval total near the 10-year average of 56. The approval total for 2025 is very close to the 61 new molecular entities and novel biologics approved in 2024.
Saol Therapeutics cannot conduct a new clinical trial of SL1009 in ultra-rare mitochondrial disorder, but aims to answer the FDA's complete response letter with new looks at available data.
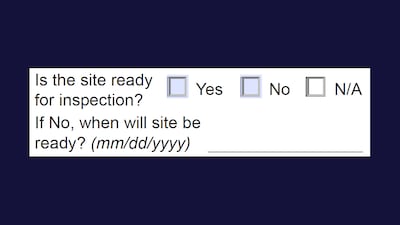
Facilities that are not ready for an inspection can lead to delays in the FDA’s site assessment and leave little time to resolve deficiencies ahead of a user fee goal, CBER compliance office Director Melissa Mendoza said.
A cluster of CRLs for rare disease applications based on one trial plus confirmatory evidence may represent a shift away from the regulatory flexibility that had come to characterize ultra-rare drug development
US FDA’s rejection of Biohaven’s troriluzole is a huge disappointment for patients with SCA and the company. But it is also a notable example of how communication strategies are changing now that FDA is making its ‘complete response’ letters public.
Meeting minute clarifications also are undergoing some modifications as part of Tidmarsh’s plans to increase efficiency and reduce workload with hiring slow going.
Recently released CRLs for unapproved products contain recommendations for new studies, including design element details and advice to consider in different submission pathways.
The agency identified deficiencies in efficacy evidence intended to support results from a single adequate and well-controlled trial for orphan applications, including weaknesses in animal data and insufficient quantity and quality of biomarker data.
The Pink Sheet breaks down the data on the 89 complete response letters recently released by the FDA, including by type of application and problems noted.
Saol cannot finance the new trial of its ultra-rare disease drug that the FDA required in a complete response letter, but hopes to leverage a 12-year history of collaboration with the agency to devise another path forward, company executives tell the Pink Sheet.
The FDA's release of CRLs for unapproved products could bring a court challenge, but for now, sponsors should expect all past and future letters will be made public, experts said.
Pink Sheet editors discuss the recent departure of a noted vaccine expert and many others from FDA advisory committee rosters, as well as the details and potential impact of the FDA’s release of dozens of complete response letters for unapproved products.
The US FDA’s unprecedented publication of 89 recent CRLs for unapproved products comes with a promise to release future letters more promptly.