Italy
Antibiotics that tackle antimicrobial resistance are to be automatically included in Italy’s Innovative Medicines Fund, which is designed to provide quick access to innovative medicines.
The Council of the EU has not taken forward a proposal from the European Parliament that would require companies to disclose the transfers of value they make to health care professionals and health care organizations – lawyers weigh in on the diverging proposals.
Payers and health technology assessment bodies in the Netherlands, Germany and Italy are either unwilling to use real-world data in assessments or cannot due to their existing frameworks, say representatives from Gilead Sciences and Autolus Therapeutics.
The world’s first CRISPR gene editing therapy, Casgevy, has been made available to patients with sickle cell disease in England, adding to access arrangements in the US, Austria, Bahrain, Germany, Luxembourg, Italy and Saudi Arabia.
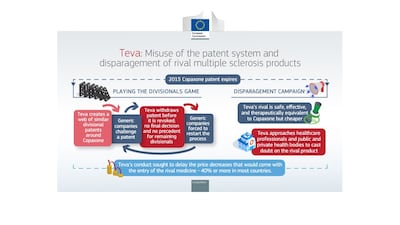
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.
The COSIsiFA initiative includes a new independent website, regular newsletters, a six-monthly bulletin, and training courses to help promote the appropriate use of medicines.
Casgevy, the world’s first CRISPR gene editing therapy, is the second drug to be accepted onto a managed access scheme via England’s Innovative Medicines Fund, offering a new treatment for patients with transfusion-dependent beta thalassemia.
Scotland’s health technology assessment body has agreed to reimburse Chiesi’s Elfabrio for Fabry disease, mirroring the decision from several other European countries – however, France turned down the rare disease drug, while Germany questioned its benefit.
Did Samsung Bioepis and Biogen choose early US access for their Lucentis biosimilar in exchange for postponing launch in other markets? That’s what Italy’s competition authority believes, as it investigates the biosimilar sponsors and originator companies Genentech and Novartis.
J&J says it has “exhausted all current viable avenues” to get its antidepressant nasal spray Spravato reimbursed on England’s National Health Service, after NICE decided against re-appraising the drug following numerous funding rejections.
Adult immunization programs can save “billions” for society, but their value is underestimated because of challenges around measuring broader value evidence and a lack of incentives for companies to collect this data, says the Office of Health Economics.
France is set to become the first country in Europe to fund Imcivree for a second – and as yet unapproved – indication. Meanwhile, England has agreed to reimburse the drug for its approved indication as did Germany earlier this year. Reimbursement discussions are underway in the rest of Europe.
Companies operating in Italy could face heavy fines if they fail to comply with a new law on disclosing the transfers of value they make, either directly or indirectly, to health care professionals and health care organizations.
US, EU, global authorities share experiences, insights, advice for industry on remote alternatives even after inspectors can resume travels.
Russia’s Sputnik V vaccine gets the all-clear in India as the COVAX initiative hits the shortages speed bump. Also, two more antibody treatments are OKd for use in COVID-19 in the UK.
Once a major source of parallel exports because of its low drug prices, Italy is now taking steps to reduce medicine spending by targeting the burgeoning parallel import market.
Ukraine has established a system for issuing emergency use authorizations for COVID-19 vaccines that recognizes authorizations granted by other world regulators.
Drug companies have their last chance to negotiate which documents and information Italy’s regulator, AIFA, will be entitled to request of them when they submit pricing and reimbursement applications.
The UK government is proposing to take the "very unusual step" of making COVID-19 vaccines available before they are licensed. The international COVAX initiative is now supporting nine vaccines, and the EU and AstraZeneca have firmed up a vaccine purchase agreement.
Italian medicines agency chief Nicola Magrini recounts the problems AIFA faced and the lessons it learned through its work at the height of the coronavirus outbreak in Italy.