Business Strategies
Public Company Edition: Veradermics grossed $294.8m, while Eikon brought in $381m, AgomAb grossed $200m and SpyGlass raised $150m, bringing this year’s US IPO count to five. Also, Adlai Nortye grossed $140m in a PIPE, while other companies cut jobs and programs.
Positive results from a randomized study in healthy volunteers mark a key milestone for Alvotech’s proposed Entyvio rivals AVT80 and AVT16.
In contributed commentary, Susan Levy, SBL Consulting Group founder and principal, discusses likely health and wellness market developments which would reflect a steady shift toward more accessible, consumer-centric models of care complementing traditional healthcare.
A biosimilar collaboration between Kashiv and Saya aims to expand access to affordable supportive oncology therapies in Mexico and 10 additional Central America and Caribbean markets.
Slovenia’s Krka is ramping up capital spending to expand global manufacturing capacity, betting that industrial scale and supply resilience will drive its next phase of growth.
The antibody-drug conjugate pipeline has more than doubled to 895 candidates since 2023, with DNA topoisomerase I overtaking HER2 as the dominant target.
The drugmaker’s sales grew by more than 40% for the fourth quarter and fiscal year 2025, with tirzepatide accounting for more than half of annual sales.
Hawaii proposes age-restricted sales but, unlike similar bills also filed in Alaska, Massachusetts, Michigan and Washington legislatures, would require behind-the-counter storage to limit consumer access in stores.
The drugmaker announced its fourth quarter and full-year results for 2025, highlighting significant revenue opportunities ahead across indications.
Teva is lining up Europe as the next regulatory market for its long-acting injectable olanzapine, following its US filing late last year, as the company looks to build a global schizophrenia franchise.
With capital being recycled, tariffs settling and interest rates softening, 2026 could provide more investment and growth opportunities in pharma and medtech, with AI giving a tailwind, say Taylor Wessing’s Ross McNaughton and Sarah Cole.
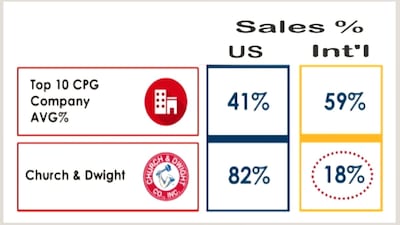
C&D starts 2026 with expectations to boost sales of latest acquisition, Touchland scented hand sanitizer, after halting sales of Flawless hair removal/nail care and Waterpik showerhead products and selling Spinbrush power toothbrush brand as well vitafusion and L’il Critter gummy supplement lines.