Gynecology & Urology
Results of survey of 986 oral contraceptive users 15 to 45 years old published online in JAMA Network Open show OTC access drove a 31.8 percentage-point increase in women shifting from no contraceptive method to an effective method.
“Women’s health continues to be ignored, underfunded, and sidelined. Too many women still die from preventable causes or live in poor health. That must change,” says Bill Gates.
“We continue to grow the brand. It was slower than initially anticipated or forecasted in basis, but I'm pleased with how it's developing,” says CEO Patrick Lockwood-Taylor of Opill performance.
IMMA’s transvaginal at-home ovarian stimulation monitoring system using ultrasound and AI image analysis increases the chances of early IVF success. It won the Biomed Israel 2025 IVD start-up showcase award. Further potential applications could follow, cofounder/CEO Beatrice Chemla said.
IMMA’s transvaginal at-home ovarian stimulation monitoring system using ultrasound and AI image analysis increases the chances of early IVF success. It won the Biomed Israel 2025 IVD start-up showcase award. Further potential applications could follow, cofounder/CEO Beatrice Chemla told In Vivo.
The Lioness non-surgical silicon ring implant is designed to put an end to pre-term births, sparing maternal anguish and saving health system costs. PregnanTech won the Biomed Israel 2025 medtech start-up award, and Limor Sandach told In Vivo how a non-digital technology beat off stiff competition.
The Lioness non-surgical silicon ring implant is designed to put an end to pre-term births, sparing maternal anguish and saving health system costs. PregnanTech won the Biomed Israel 2025 medtech start-up award, and Limor Sandach told In Vivo how a non-digital technology beat off stiff competition.
Mirvie launched Encompass, a blood test to help identify women over age 35 who are at moderate risk for preeclampsia, and will conduct additional studies to support reimbursements from payers.
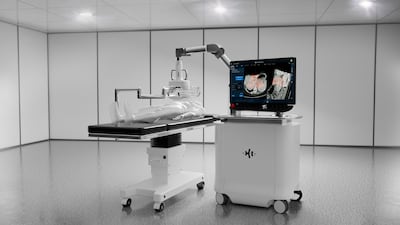
SS Innovations announced plans to file a de novo application with the US FDA for its SSi Mantra 3 surgical robot, already approved for marketing in six countries. The Florida-based firm also plans to pursue the CE mark for European commercialization.
HistoSonics, which developed a noninvasive technology to destroy tumor cells, reports 90% local tumor control at 12-month follow-up in the #HOPE4Liver Trial. The Edison System, cleared by the US FDA in late 2023, is also being evaluated for kidney and pancreatic tumors. CEO Mike Blue said the medtech is financially secure but watching public markets as it considers an IPO.
Medtech Insight sat down with Intuitive Surgical CEO Gary Guthart at the recent LSI USA conference to discuss the full launch of the new da Vinci 5 robotic system and planned digital enhancements. Guthart also offered his views on health care interoperability, AI regulation, outpatient surgeries, autonomous robots, and how the company is harnessing technology to shape the future of robotic surgery.
Several new drugs that are yet to be approved anywhere in the world are now under review by the European Medicines Agency for potential pan-EU marketing authorization.