New Products
Futura Medical "delighted" with early feasibility study results for topical OTC treatment WSD4000.
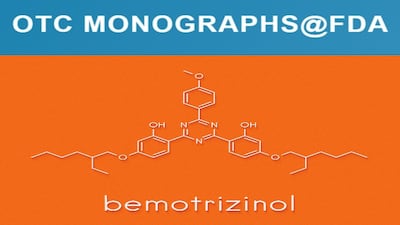
FDA’s proposed order follows its review of OMOR DSM-Firmenich submitted showing bemotrizinol, at concentrations up to 6%, is generally recognized as safe and effective and can be added as an active ingredient to sunscreen monograph.
The trophies have been presented at the OTC Marketing Awards 2025 - but what did the judging panel have to say about the winning and highly-commended entries?
Winners across 12 categories were announced at the OTC Marketing Awards 2025 on 20 November, with Opella crowned OTC Company of the Year.
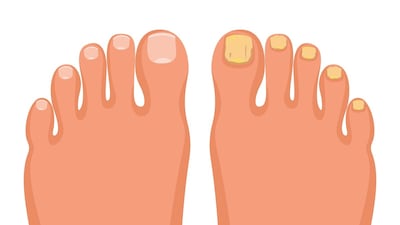
Karo will take on responsibility for the marketing, distribution and sales of Moberg’s topical onychomycosis treatment, MOB-15, under the Lamisil brand in 19 European countries.
The shortlist has been revealed for the upcoming 30th Annual OTC Marketing Awards, which takes place on Thursday 20 November in London, UK.
UK health & beauty retailer Boots has partnered with IBSA to launch a "first of its kind" orodispersible non-prescription sildenafil option.
Thanks to a successful reclassification by Maxwellia, UK consumers have a new option for relief from musculoskeletal pain.
Haleon adds herbal pain relief to Voltaren topicals line, Bayer launches herbal expectorant in time for autumn and Angelini creates a throat spray that meets German Society for General Practice and Family Medicine guidelines.
UK children will be able to clean their teeth with Paw Patrol themed Aquafresh toothbrushes and toothpastes following launches from Haleon.
Signos links users to Dexcom’s Stelo for glucose readings and historic trends. The app is available in iOS and Android from the firm’s website, where it’s priced at $139 for three-month plan and $129 for a two-month plan, or online app stores.
Eyelids have the "thinnest, most sensitive skin on the body" and are "highly prone to dehydration," which is why Scope has launched new Optase Life Sensitive Eye Daily Renewal Cream.
Terclara - Moberg’s proprietary topical formulation of terbinafine for fungal nail - has established itself as the market leader in Norway just months after its OTC launch. The firm is now plotting its expansion to additional EU markets.
Find out how to enter the OTC Marketing Awards before the 1 September entry deadline.
Navamedic has launched Eroxon in Danish pharmacies giving men in the country OTC access to an ED treatment for the first time.
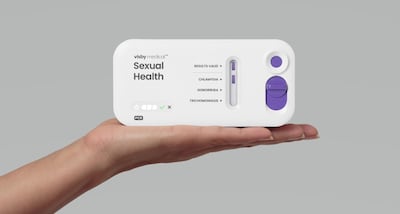
The Visby Medical Women’s Sexual Health Test is the first over-the-counter test cleared by the US Food and Drug Administration to detect chlamydia, gonorrhea and trichomoniasis. It delivers results in about 30 minutes.
Pirilieve Hayfever Relief Tablets contain 120mg fexofenadine hydrochloride, which the firm notes is an active ingredient that was “previously only available behind pharmacy counters.”
Consumer uptake of Eroxon - the first FDA-approved OTC ED treatment available in the US - has been slower than expected, according to Haleon, which says its working with retailers to build awareness.
Start-ups and individuals working on innovative consumer health concepts, in categories such as pain relief and dietary supplements, have the opportunity to access Haleon's resources and expertise to make their ideas a reality.
Voltarol One A Day Muscle Pain Relief Medicated Plaster provides up to 24 hours relief from pain and inflammation, according to manufacturer Haleon.