Policy & Regulation
Germany’s Expert Committee for Prescription (SVA) has unanimously rejected the proposed switch of the controversial painkiller metamizole from prescription to OTC status.
Recommendations to mitigate attrition among clinical research and drug manufacturing investigators at the FDA have not been implemented, and a Strategic Workforce Plan aimed at addressing recruiting, retention and training challenges has been shelved, the Government Accountability Office said.
Featuring: Prevention‑driven reforms in Vietnam; surging demand for OTC and herbal based interventions in China; Singapore updates its health data infrastructure; regional regulatory harmonization across Australia, Singapore and Indonesia; and WHO sets formal direction for TCIM.
Umbrella branding in OTC medicines supports responsible self‑care by helping consumers navigate choices, build trust and confidence, and access reliable health information, countering regulators’ long‑standing focus on risks rather than benefits, says PAGB CEO Michelle Riddalls.
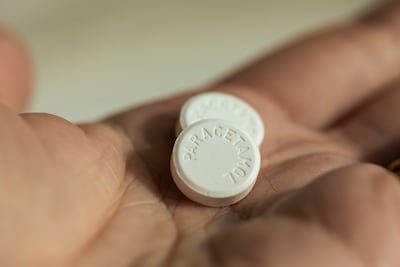
A new Lancet meta-analysis finds no evidence that paracetamol use in pregnancy increases the risk of autism, ADHD, or intellectual disability, decisively refuting claims made by US President Donald Trump and reaffirming regulatory guidance that the drug remains safe when used as directed.
HBW Insight speaks to ex-Perrigo ESG expert Steve Ramus about the state of sustainability in consumer health and where we might be heading in the near future. Part one of two.
Huge opportunities for OTC therapies loom in India, as consumerization brings structural reset in healthcare. Success stories on Rx-to-OTC transition augur well for pharma, but a predictable regulatory roadmap will be pivotal for sustainable growth.
Industry associations in the UK, Germany and Greece expect 2026 to be the year self‑care policy is translated into day‑to‑day system change. They highlight opportunities in pharmacy, digital tools and regulatory reform, but warn that progress depends on political timing and regulatory agility.
A new report by Bayer, GSCF and BCIU predicts that 2026 may be pivotal for embedding self‑care into global health policy, as progress toward universal healthcare has stalled and urgent, evidence‑driven advocacy is needed to ensure self‑care becomes a core, scalable component of health systems.
GSCF, AESGP and ILAR agree that 2026 will see self‑care translate into more tangible system‑level action, propelled by prevention, regulatory updates and more assertive efforts to counter misleading claims.
Consumer health companies can help accelerate the shift to prevention as employers and through innovation and cross-sector partnerships, unlocking a potential £42bn in savings for the UK National Health Service.
FDA updates pair of guidance documents relaxing its posture on how it regulates general wellness devices and clinical support software. Industry mostly welcomes changes, agreeing with agency’s view that lighter regulation will help spur innovation.
The South African Health Products Regulatory Authority is calling on manufacturers of food supplements marketed for children containing zinc picolinate and/or selenium to withdraw these products from the market within six months of the notice.
“Our sense is that companies are currently focused on timing, scope and execution. For example, knowing when a final order might issue … We also imagine that formulators are scrutinizing the proposed list of permitted combinations, the 6% cap, and the permitted dosage forms,” say ArentFox Schiff att
Germany’s Expert Committee for Prescription meets on 20 January to discuss the Rx-to-OTC switch of controversial painkiller metamizole, which has been banned in a number of countries around the world because of the risk of agranulocytosis.
Commissioner Martin Makary told staff that more than 400 new people are being onboarded, although where they will be placed is unclear.
AI is rapidly reshaping consumer health advertising across Europe, supporting creative work, compliance checks and regulatory oversight, according to a recently published report by UK consumer healthcare industry association, PAGB.
Agency says FY 2026 Tier 1 OMOR fees are $587,529 and Tier 2 fees are $117,505. The fees, due to FDA when an OMOR is filed, aren’t included in OMUFA target revenue calculation for each fiscal year based entirely on annual facility registration fees.
During webinar hosted by Sedgwick, regulatory experts discussed how firms can optimize engagement with FDA, especially when it comes to communicating recalls and corrective actions.
Former agency officials now representing industry worry that a deregulatory bent could be driving "Simple Reform" plan to merge all medical product and clinical research inspectorates and that specialist expertise gained in 2017 "Program Alignment" initiative will be reversed.