HBW Insight
Oakland, Calif.-based E.l.f. Beauty Inc.’s reported sales in the fiscal third quarter soared 38% to $489.5m and its net income reached $39.4m, more than double what it achieved in the same period a year ago.
AI advisers could eventually guide consumers toward health products based on personalized needs rather than traditional marketing, IQVIA Consumer Health suggests. But brands will still need to balance data‑driven recommendations with the human appeal of in‑store discovery and self‑care experiences.
Germany’s Expert Committee for Prescription (SVA) has unanimously rejected the proposed switch of the controversial painkiller metamizole from prescription to OTC status.
Agency exercises enforcement discretion on requirement for products to be labeled as containing artificial colors or dyes when those aren’t native to foods or ingredients, including added colors made from natural sources.
The Estee Lauder Companies' ‘Beauty Reimagined’ initiative is paying off a year after launch, with expanded distribution of brands and ‘breakthrough’ innovations, the firm says during a Feb. 5 fiscal second quarter earnings presentation.
In contributed commentary, Susan Levy, SBL Consulting Group founder and principal, discusses likely health and wellness market developments which would reflect a steady shift toward more accessible, consumer-centric models of care complementing traditional healthcare.
French firm's new supplement combines seven ingredients to offer relief from common menopause symptoms.
Recommendations to mitigate attrition among clinical research and drug manufacturing investigators at the FDA have not been implemented, and a Strategic Workforce Plan aimed at addressing recruiting, retention and training challenges has been shelved, the Government Accountability Office said.
Featuring: Prevention‑driven reforms in Vietnam; surging demand for OTC and herbal based interventions in China; Singapore updates its health data infrastructure; regional regulatory harmonization across Australia, Singapore and Indonesia; and WHO sets formal direction for TCIM.
The Estee Lauder Companies, Inc. pleaded guilty to two counts of violating a Canadian Act that had required it to correct missteps related to selling products containing PFAS.
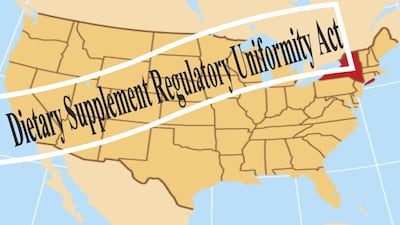
“Dietary Supplement Regulatory Uniformity Act” would prohibit states from adding requirements and rules for supplement manufacturing and sales on top of FDA regulations.
The latest round of updates to the European Commission's Novel Food Catalogue determine whether authorization is required for supplements containing certain botanical extracts.
For Super Bowl LX on Feb. 8, e.l.f. Cosmetics will air a telenovela-inspired campaign with Academy Award-nominated actress Melissa McCarthy, as Dove features 30-second spot ‘The Game is Ours,’ to inspire body confidence in young girls. Separately, Manscaped launches first Super Bowl ad.
Hawaii proposes age-restricted sales but, unlike similar bills also filed in Alaska, Massachusetts, Michigan and Washington legislatures, would require behind-the-counter storage to limit consumer access in stores.
Umbrella branding in OTC medicines supports responsible self‑care by helping consumers navigate choices, build trust and confidence, and access reliable health information, countering regulators’ long‑standing focus on risks rather than benefits, says PAGB CEO Michelle Riddalls.
UK rejects proposed health claim from AFT Pharma, “Green kiwifruit powder contributes to the maintenance of normal defecation.”
The European Commission’s recent environmental omnibus failed to address industry concerns on wastewater cost allocations, and legal pathways are expected to be the way forward.
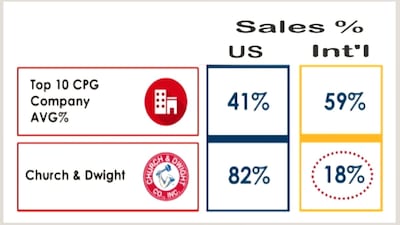
C&D starts 2026 with expectations to boost sales of latest acquisition, Touchland scented hand sanitizer, after halting sales of Flawless hair removal/nail care and Waterpik showerhead products and selling Spinbrush power toothbrush brand as well vitafusion and L’il Critter gummy supplement lines.
A round-up of the latest European people moves: Angelini hires external affairs chief; Pierre Fabre appoints impact and reputation officer; Boots changes leadership at the top.
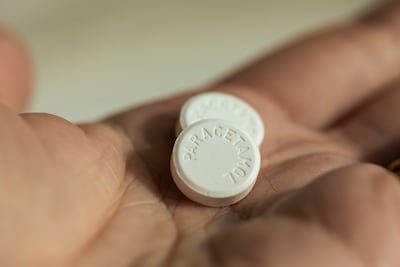
A new Lancet meta-analysis finds no evidence that paracetamol use in pregnancy increases the risk of autism, ADHD, or intellectual disability, decisively refuting claims made by US President Donald Trump and reaffirming regulatory guidance that the drug remains safe when used as directed.