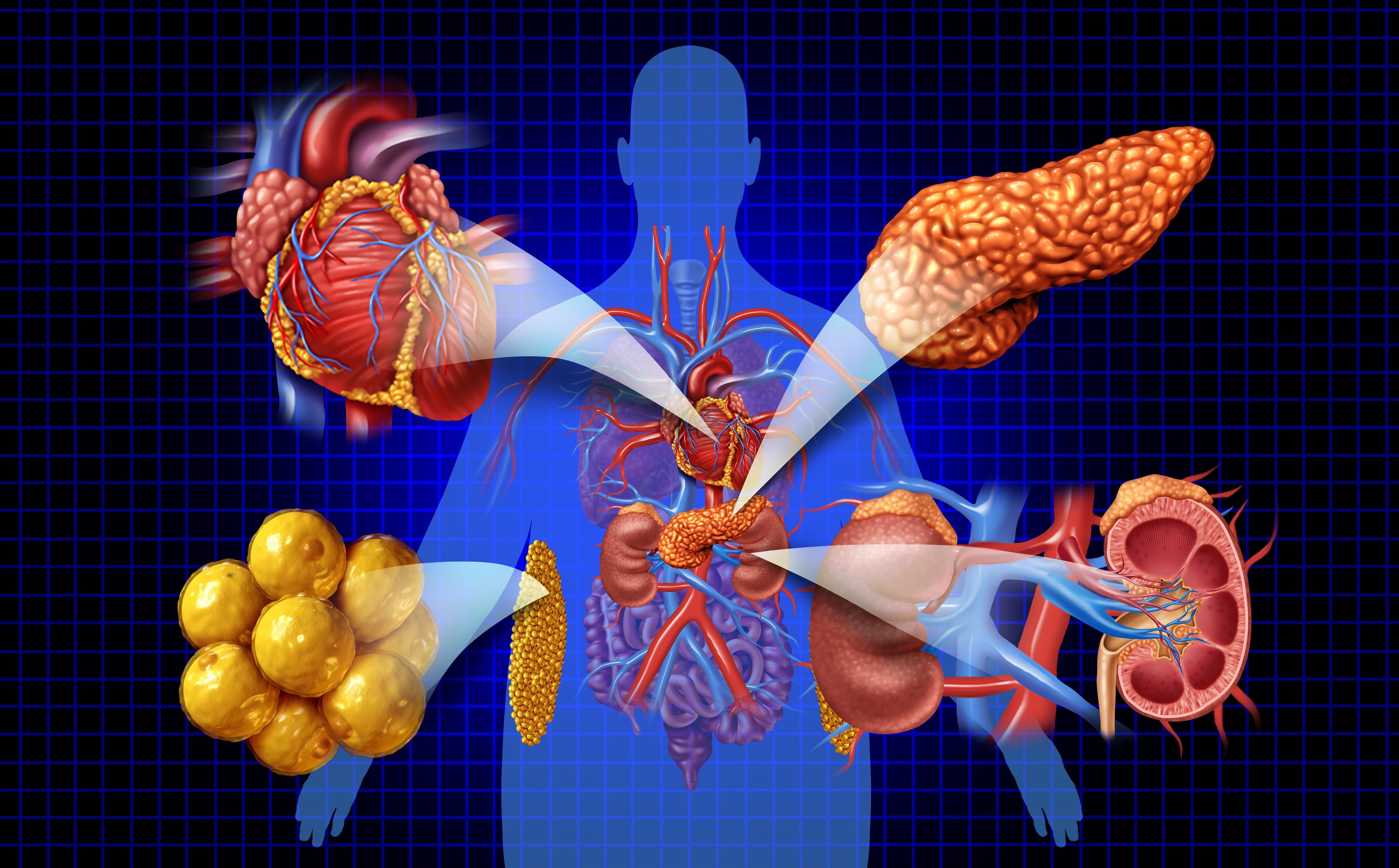
US FDA CNPV Reviews Clarified: Center Director Approval Recommendations Not Binding, HHS Says
However, their recommendation, coupled with those of the Commissioner’s National Priority Voucher Review Council, likely will be a source of pressure on scientific reviewers and the division or office directors who are the signatory authorities on applications.