Archive
Editor’s note: This is your final call to participate in the survey to better understand our subscribers’ content and delivery needs. The deadline is 20 September.
Editor’s note: We are conducting a survey to better understand our subscribers’ content and delivery needs. If there are any changes you’d like to see in the coverage topics, content format or the method in which you receive and access Generics Bulletin, or if you love it how it is, now is the time to have your voice heard.
Bringing in sales that translated to nearly $1.5bn last year, Japan’s Towa was feeling upbeat after a challenging few years, both for itself and the broader Japanese generics market. However, its financial guide for the next 12 months has left investors feeling cold.
Introducing the all-new Citeline News and Insights app, providing seamless access to all your favorite award-winning publications. Explore the latest articles from Pink Sheet, Scrip, In Vivo, Medtech Insight, Generics Bulletin and HBW Insight, all in one stylish, intuitive and user-friendly platform.
More than a decade after a renewed bid to take control of Taro failed, Sun Pharma is set to take full control of the Israeli-based derma specialist, which has seen material growth and much controversy in the US under Sun’s ownership.
Norwich brought suit against the US Food and Drug Administration in its bid to get final approval for its Xifaxan ANDA product with patent-protected indications carved out, following an earlier patent-infringement decision. Now, a court in Washington DC has had its say.
Since its launch in 2018, Adalvo has grown in the B2B pharma space through a four-pillar strategy and has completed over 600 transactions. It aims to be the leading European player in this space by 2025.
Senderra Specialty Pharmacy is the latest service provider to announce expanding access to adalimumab products, making five Humira biosimilars available across its network.
Celltrion has doubled down on its alliance with Rani Therapeutics for oral pill versions of biosimilars by adding adalimumab to an existing collaboration covering ustekinumab.
Pfizer’s $43bn agreement to acquire Seagen would layer its portfolio and pipeline with vastly complex antibody-drug conjugates – a class of drug that both parties feel might be almost immune to biosimilar competition.
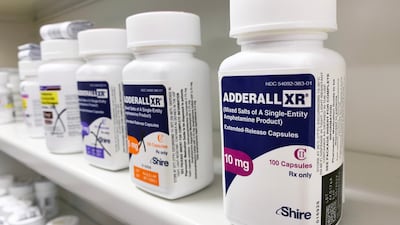
Teva, one of the largest manufacturers and suppliers of branded and generic Adderall, the renowned treatment for ADHD and narcolepsy, has responded to claims.
Two-year-old summary judgments have finally been addressed by a US district court.
As the Fight The Fakes Alliance holds its fifth annual “Fight The Fakes Week,” the IGBA has signed to become a new member of the association as it calls for fresh action to prevent falsified medicines from reaching patients.
Medicines For Europe and Czech association CAFF demand EU and national reforms to help drug makers ensure supply and patient access, particularly in Central and Eastern Europe.
With glucagon-like peptide 1 products to treat diabetes and obesity continuing to gather pace, Sandoz has expressed confidence that it is capable of bring such complex products to market using its vast array of in-house capabilities.
Biogen is looking for its ranibizumab biosimilar Byooviz to make a splash in 2023 as it continues to forecast falling biosimilars sales for 2022.
Medicines for Europe has urged the European Commission to exempt pharmaceutical suppliers, particularly those of biologic and antibiotic medicines, from any perspective energy rationing in the region, in line with new public guidelines.
The director-general of the European Commission’s recently-formed Health Emergency Response Authority addressed delegates to off-patent association Medicines for Europe’s 2022 annual conference, noting that it was preparing a report for post-summer as the HERA assessed its priorities.
The Union for International Cancer Control has established the Access to Oncology Medicines Coalition to facilitate access to cancer drugs in low and lower-middle-income countries.
As the conflict in Ukraine moves into its second month, efforts to provide essential medicines to those affected are continuing across the off-patent industry, with regional association Medicines for Europe urging additional action to allow the safe production and transportation of supplies.