Recalls
SC Johnson & Son, Inc.’s Babyganics brand issues voluntary recall of Totally Tropical scented mineral sunscreen rollerball and continuous spray formulas after testing was found ‘unacceptable,” though the brand says there is no risk to health or safety.
Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”
The US FDA should use guidance or rulemaking to clarify MoCRA provisions related to adulteration, Amin Wasserman Gurnani attorney Angela Diesch suggested at the Independent Beauty Association’s Cosmetics Convergence Spring Symposium. Attendees also sought her take on whether the agency’s new recall authority is likely to spell an increase in cosmetic product recalls.
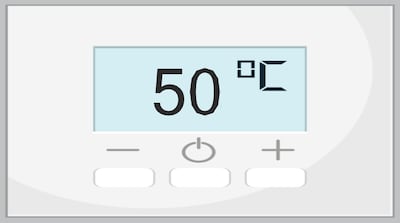
As stability temperature for study of 66 products, it says 50°C is recognized as reasonable temperature product may be exposed to during distribution and handling and is accepted temperature used by pharma industry for accelerated stability studies of at least three months.
Higher costs and interest rates due to inflation as well as higher credit card debt point toward consumers spending less in areas including health, personal and household care products. C&D is “well-positioned for this trade down” with 40% of its portfolio in value products, says CEO Matthew Farrell.
Recall starts after products across OTC indications, including at-home pregnancy and marijuana tests, “were stored outside of labeled temperature requirements” and “inadvertently shipped to certain stores” in 23 states.
The agency plans to move cosmetics regulation and color certification functions out of the Center for Food Safety and Applied Nutrition and into the Office of the Chief Scientist. The Personal Care Products Council and Independent Beauty Association provide their initial reactions.
Senate HELP chair Patty Murray says she will ensure FDA implements cosmetics reform correctly and “effectively” uses enforcement tools. EWG exec says previous standards didn’t address “chronic harm caused by a known carcinogen like benzene.”
Following reviews prompted largely by formula supply crisis, FDA will move Center for Food Safety and Applied Nutrition and commissioner’s Office of Food Programs and Response to Human Foods Program headed by deputy commissioner. Office of Regulatory Affairs' structure also changing “to support the FDA organization as a whole,” says Commissioner Califf.
Companies have one year to comply with US FDA registration and product listing requirements under the Modernization of Cosmetic Regulations Act of 2022, part of the $1.7tr omnibus spending package signed by President Joe Biden on 29 December. Trade groups applauded the historic milestone and the legislation’s departure from previous proposals, including the elimination of user fees.
The firm has instructed retailers to remove, and consumers not to use, selected lots of dry shampoo aerosol products from Dove, Nexxus, Suave, TIGI and TRESemme. The voluntary action follows Unilever’s recall of Suave aerosol antiperspirants in March, also due to potential benzene contamination.
The agency is calling for an ISO 17025-accredited or equivalent partner to screen selected drug products for benzene, following a rash of sunscreen, antiperspirant and other OTC drug product recalls which triggered a rush on testing laboratories, as well as class action filings across the US.
Firms says products recalled were “inadvertently shipped to certain stores” after “being stored outside of labeled temperature requirements." Recalls include oral products and nasal sprays, oral care products, sunscreen and other topicals, supplements and variety of personal care SKUs.
With higher demand a norm, OTC sanitizer firms should do the same with scrutiny of their supplies of alcohol and other ingredients. “The market itself will always be much higher than a pre-COVID level as it’s a consumer product that is going to be used on a more regular basis,” says Ed Wyszumiala, head of US Pharmacopeia’s verification program.
The SC Johnson brand is providing full refunds to consumers who purchased the chamomile verbena Bubble Bath products, which may contain a bacterium that is potentially harmful to the immunocompromised and those with broken or irritated skin, “such as diaper rash.”
Samples of two Suave 24-Hour Protection Aerosol Antiperspirants tested positive for “slightly elevated levels of benzene,” leading Unilever to recall all lots of the products that remain in circulation after the line’s discontinuation “for business reasons” in October 2021.
Final guidance describes in greater detail than draft published in 2019 the agency’s expectations for marketers and other businesses’ responses when they need to recall FDA-regulated products.
Procter & Gamble is recalling more than 30 aerosol dry shampoo and conditioner formulas from the US market under the Pantene, Aussie, Herbal Essences, Waterl<ss, Old Spice and Hair Food brands, after an internal portfolio review found low levels of benzene contamination.
FDA makes withdrawal effective on 31 December, also the deadline for firms manufacturing or compounding alcohol-based hand sanitizer products, and making alcohol for those products under temporary guidances, to cease production.
J&J says the recall decision was made out of an abundance of caution, maintaining that daily exposure to benzene at levels detected in J&J sunscreen products “would not be expected to cause adverse health consequences.”