Diabetic Care
A warning letter from the US FDA citing concerns of some Abbott continuous glucose monitors will not stop the company from launching a novel diabetes sensor later this year as planned.
BioStem Technologies’ buyout of BioTissue Holding’s surgical and wound-care business adds cryopreserved and sterile technologies, Cryotek and SteriTek, and a direct sales force focused on acute care settings.
Withings announced at CES that its health monitoring app will integrate data from Abbott’s OTC CGM Lingo starting in Q1.
Timed to debut during this week’s CES 2026 technology conference in Las Vegas, the new feature within Abbott’s Libre glucose monitoring software uses generative artificial intelligence to predict how food choices may affect glucose levels.
Last year was a busy one for the medtech industry, with major advances in technology as well as big changes at regulatory agencies. Get a peek at the stories our readers couldn't miss.
MiniMed flagged launch-related challenges in its IPO filing, saying production of its Simplera CGM is scaling more slowly than expected due to underperformance in initial high-volume automated manufacturing.
The system combines a durable rechargeable transmitter with a two-year life span and a replaceable single-day sensor. The product is intended to address pain, inconvenience and adherence challenges associated with multiweek CGM use.
The US FDA is launching a pilot to promote access and safety to digital health devices. Developed by the agency’s device center, the pilot will evaluate a new, risk-based enforcement approach for certain types of digital devices to treat several conditions.
New rule in effect Jan. 1 follows OIG call for CMS to use competitive bidding to adjust payments for continuous glucose monitors after finding that Medicare was paying above supplier costs and retail prices.
US Medicare payments for CGMs and supplies in the year to June 2023 exceeded suppliers’ acquisition costs by $377m, says new OIG report
Abbott has initiated a medical device correction for about 3 million FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors in the US after internal testing detected a risk the device may produce inaccurate glucose readings.
Brain-computer interfaces advance toward trials and commercialization, Oura pushes for FDA-cleared blood pressure monitoring, and regulators weigh AI’s expanding role in mental health and diagnostics amid rising safety concerns.
Tandem Diabetes Care is positioning for growth in 2026 with the launch of its Mobi Tubeless pump following FDA clearance of its Android-compatible Mobi app while navigating multiple recalls during what CEO John Sheridan called a transformative year.
Viome’s CEO To Expand Into Clinical Diagnostics ‘For Which There Are No Solutions’ With AI, RNA Test
Viome Life Sciences accelerates its push into clinical diagnostics with studies in colorectal cancer and a major partnership with Microsoft to scale its molecular data analysis platform. Viome leverages RNA analysis and AI to detect disease at the molecular level and personalize preventive health.
Wearables innovator Oura has launched a profile study aimed at helping the company secure FDA clearance for a blood pressure feature for its smart ring. Study participants will answer questions while wearing the ring, which the company plans to use to support the feature’s efficacy.
Some of the first manufacturers to receive Medicare coverage for their breakthrough devices through the Transitional Coverage for Emerging Technologies pathway praise CMS’ engagement and timelines in the process, despite some challenges around post-launch data collection.
Though the advance of remote medical device technology allows for better at-home care, it comes with challenges and risks, says AdvaMed, which has published a safety bulletin guiding stakeholders on the operation of remote devices.
Fresh off de novo clearance, Biolinq plans to launch its needle-free CGM for type 2 diabetes not on insulin in early 2026. Pricing has not been disclosed, but “won’t be too far apart” over rivals Abbott’s Lingo and Dexcom’s Stelo CGMs, CEO Rich Yang said.
Dexcom has endured recalls, layoffs and leadership change, but interim CEO Jake Leach said the company remains committed to innovating next-gen devices.
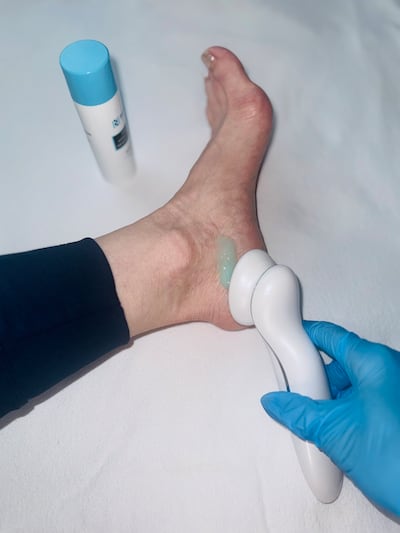
Rapid Nexus hopes its regenerative wound-healing system can ease the burden of diabetic foot ulcers and amputations. Backed by early clinical data and $3.8m in seed funding, the start-up is targeting the VA as a launchpad into a $6.5bn US market, with FDA clearance expected later this year.