Respiratory
The US FDA has granted clearance to eMurmur, broadening the use of its AI-powered software platform. The innovative system identifies primary heart sounds to detect abnormal murmurs as they occur.
Medtech Insight spoke with CTA board member Carlos Nunez and AARP’s Andy Miller about what to expect at this year’s CES.
Aerogen, which is Ireland’s largest native medical technology firm, is in clinical trials for Aerofact, a nebulizer aimed at aiding premature infants' breathing. The technology promises easier administration of surfactant, with potential commercial rollout projected in three to four years, if succes
US FDA inspections of three Philips manufacturing sites earlier this year resulted in a September warning letter that claimed the company was not in conformity with current good manufacturing practices. Philips says it is addressing the agency’s concerns and working to enhance its quality systems.
Medtech Insight sat down with Resmed’s CMO Carlos Nunez at HLTH USA to discuss the sleep giant’s continued push from medtech into healthtech and its efforts to fix what he calls a “broken pathway” to diagnosis and treatment for millions with undetected sleep apnea.
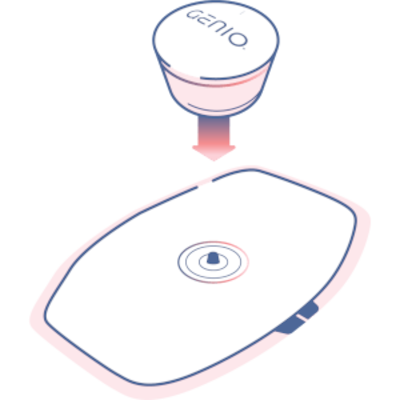
After receiving FDA clearance for its Genio sleep apnea implant, Nyxoah plans a major US rollout despite a patent suit from rival Inspire Medical. Genio offers bilateral nerve stimulation as a CPAP alternative, with strong trial results.
Draeger Medical has recalled certain SafeStar and TwinStar ventilation filters after reports of serious injuries caused by misleading carbon dioxide readings.
Apreo Health CEO Karun Naga talks to Medtech Insight about the company’s intentions for its Series B funding. The Breathe-3 clinical trial and early commercialization activities, involving physician education, are top priorities.
Philips Respironics has updated instructions for use for three ventilators that were included in a May 2024 recall. The company also added three potential injuries and one death to the original number.
CDX is eying applications for its pump platform that go beyond ECMO. The company’s CEO says smart pumps that can be fine-tuned to patient volume and configuration are needed, and CDX is creating a scalable solution capable of supporting both acute and chronic lung care.
Leaders of robotic systems companies Distalmotion, Neocis and Noah Medical discussed success metrics, competition and funding. Institutional investors are focusing on utilization, procedure rates and a clear path to profitability as the IPO window reopens, BTIG analyst Ryan Zimmerman said.
After two serious injuries and one death linked to some of its ventilators, Medtronic has recalled the devices and asked customers to stop using them and find alternative means. The action comes more than a year after the company left the market.
BioWales in London 2025 showcased the efforts healthtech innovators are making to meet investors on their own turf, illustrating changing attitudes and evolving needs.
Ultrahuman seeks to “create an environment that aligns with our bodies" by linking environmental parameters collected by Ultrahuman Home to health physiology, measured by the Ultrahuman Ring Air.
“Infectious disease should be treated in the community, not in hospitals,” Alex Batchelor, Pictura Bio CEO, told Medtech Insight at the Anglonordic Life Science Conference on 3 April in London.
Medtech Insight sat down with Intuitive Surgical CEO Gary Guthart at the recent LSI USA conference to discuss the full launch of the new da Vinci 5 robotic system and planned digital enhancements. Guthart also offered his views on health care interoperability, AI regulation, outpatient surgeries, autonomous robots, and how the company is harnessing technology to shape the future of robotic surgery.
Cambridge, UK-based breath biopsy company Owlstone Medical will apply its volatile organic compound analysis platform in an attempt to develop a test for the identification and monitoring of pseudomonas aeruginosa in patients with cystic fibrosis.
The US FDA has announced seven device safety actions in recent days. The most serious, which relates to a manufacturing defect in Boston Scientific’s Accolade pacemaker, has been linked to 832 injuries and two deaths.
Anita Jackson is facing 25 years for device adulteration and a host of other charges. She says the trial and appeals court failed to consider her defense.
Hologic attains full US Food and Drug Administration 510(k) clearance for its Aptima SARS-CoV-2 assay, which reached market under an Emergency Use Authorization (EUA). Overall, Hologic’s “best-performing” molecular diagnostic business continues to grow despite the decline in revenue for COVID-19 assays and related items.