AI
A warning letter from the US FDA citing concerns of some Abbott continuous glucose monitors will not stop the company from launching a novel diabetes sensor later this year as planned.
The US FDA has published its annual international harmonization assessment for fiscal 2025, which reports on the device center’s progress in aligning medical device regulations with global standards to improve patient access to safe, innovative technologies.
Women’s health start-up Midi Health has raised $100m in a Series D financing, led by Goodwater Capital with new investors Foresite Capital and Serena Ventures. Midi, which focuses on women in midlife, said the money will support the next phase of growth.
Medtronic has agreed to acquire Cathworks for up to $585m, converting a long-standing partnership into full ownership as it expands its footprint in coronary physiology and AI-enabled cardiovascular tools.
INBRAIN unveiled a bidirectional "rice-sized" BCI chip partnership, Merck commercialization progress and new speech-decoding trial in France as it advances its graphene-based cortical interface toward commercialization, pending regulatory clearance.
Medtech Insight was invited to moderate a panel discussion with leading experts in neuroscience and AI during INBRAIN’s five-year anniversary in Barcelona, Spain. Panelists discussed the promises, perils in BCI development, neuroethics and outlook.
The US FDA has granted clearance to eMurmur, broadening the use of its AI-powered software platform. The innovative system identifies primary heart sounds to detect abnormal murmurs as they occur.
In this final part of a three-part series, Medtech Insight spoke with a neuroethicist and the first person in a trial using a BCI implant for stimulating hand movement. This story explores ethical considerations that arise when projects can no longer support patients with implanted devices.
The US FDA has published a draft guidance on clinical performance testing and evaluation for premarket submissions from manufacturers of cuffless blood pressure measuring monitors, which the agency generally regulates as class II devices.
Chatbots can churn out valuable information to patients and give much needed assistance to healthcare personnel. But their mistakes can lead to significant patient harm, which is why ECRI ranks their misuse as the top health technology hazard for 2026.
Amid the spectacle of humanoid robots at CES 2026, Swedish medical simulation company Surgical Science opted for a quieter pitch for its suitcase-sized robotic surgery simulator aimed at taking training out of the OR.
Healthtech companies seek to optimize the opportunity of AI while navigating a thicket of requirements from providers as well as regulations like the EU MDR. Andrew Davies, digital health lead at the Association of British HealthTech Industries, explained the importance of streamlining AI processes.
The US FDA has updated a pair of guidance documents relaxing the agency’s posture on how it regulates general wellness devices and clinical support software. Industry has mostly welcomed the changes, agreeing with the agency’s view that lighter regulation will help spur innovation.
After receiving the FDA nod for the first in-ear EEG device for brain monitoring, Noax plans four clinical trials in the US focused on epilepsy and is looking for audio company partners to integrate the same tech for use by consumers to monitor their brain activity, the company told Medtech Insight.
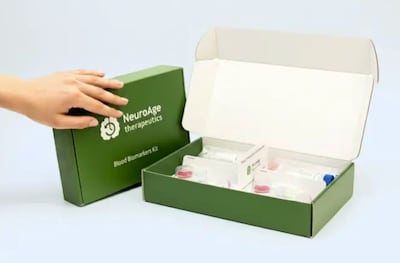
MIT-scientist turned entrepreneur Christin Glorioso founded start-up NeuroAge Therapeutics to reverse brain aging and fight dementia.
AI in medtech offers exciting potential for improving patient care and operational efficiency, but faces significant regulatory challenges. Ensuring data quality and building trust among users are essential for successful AI integration in healthcare, our expert panel said.
In this first roundup from CES, Medtech Insight shines a light on AI-powered technologies that help the aging population live healthier and stay independent longer.
The UK health care products regulatory agency is inviting industry to share its views on how artificial intelligence in health care should be regulated, with input set to shape future rules and guidance.
Makary Touts ‘AI Revolution’ In Announcing Guidance Docs On Wellness Products, Decision Support Tool
FDA Commissioner Marty Makary announced new guidance documents on wellness products and decision support tools focused on AI technology at CES in Las Vegas. The documents aim to reduce regulatory hurdles and promote innovation while ensuring public safety.
Withings announced at CES that its health monitoring app will integrate data from Abbott’s OTC CGM Lingo starting in Q1.