Cardiology
Medtronic has agreed to acquire Cathworks for up to $585m, converting a long-standing partnership into full ownership as it expands its footprint in coronary physiology and AI-enabled cardiovascular tools.
The US FDA says Abiomed has reported 22 serious injuries linked to an issue with some of its temporary heart pumps that provide support to patients with acute right heart failure.
The US FDA has granted clearance to eMurmur, broadening the use of its AI-powered software platform. The innovative system identifies primary heart sounds to detect abnormal murmurs as they occur.
The US FDA has published a draft guidance on clinical performance testing and evaluation for premarket submissions from manufacturers of cuffless blood pressure measuring monitors, which the agency generally regulates as class II devices.
Abbott started 2026 as it ended 2025 – with major regulatory breakthroughs for pulsed field ablation innovations, boosting its EP credentials in the US and EU. It also broadened its IVDs platform by adding cancer detection and screening company Exact Sciences in a deal expected to close in Q2.
Boston Scientific’s $14.5bn Deal Folds Penumbra Thrombectomy Platforms Into Cardiovascular Franchise
Subject to shareholder approval,Boston Scientific will acquire Penumbra for approximately $11bn in cash on hand and new debt, and issue 41 million shares for the remaining $4bn.
The one-time implant for patients with nonvalvular atrial fibrillation, Conformal Medical’s CLAAS, reduces stroke risk by preventing blood clot formation in the LAA and cutting the need for long-term oral anticoagulation therapy. Gore’s purchase sees it enter a $7bn market.
In a letter to its customers, Medtronic said it had also received 41 complaints indicating that the catheters were not retaining their intended shape when bent. The FDA issued an early alert about the recall in August but did not at that time assign a recall class.
Abbott, which secured CE Mark approval earlier this year, is hopeful that Volt, which can integrate with a 3D mapping system, will bring an edge over competitors. It also won FDA approval and CE mark for a heart device for premature babies, the Piccolo Delivery System.
Philips says it intends to fold SpectraWAVE’s intravascular imaging and physiological assessment technologies into its existing intravascular ultrasound ecosystem, including Eagle Eye Platinum catheters and the IntraSight imaging and physiology workspace.
Medicare's new cardiac monitoring payment updates are set to boost home monitoring adoption by easing billing processes and improving reimbursement rates. The changes may benefit providers directly, shifting control from diagnostic testing facilities and potentially reshaping the RPM market.
Xeltis secured €50m to scale and market its aXess vascular access graft, a bioresorbable scaffold that transforms into a patient’s own living vessel. With EU regulatory review underway and US trials advancing, Xeltis sees new US Medicare rules as a major opening for alternative treatments.
The US FDA is launching a pilot to promote access and safety to digital health devices. Developed by the agency’s device center, the pilot will evaluate a new, risk-based enforcement approach for certain types of digital devices to treat several conditions.
The French start-up has successfully implanted its ‘fishtail’ LVAD in a patient for 84 days, where it served as an effective bridge to transplant. Medtech Insight spoke to the company’s CEO, Louis de Lillers, to hear more about the company’s progress and his take on the evolving LVAD market.
Medtech funding in 2025 sees a trend of fewer, yet larger deals with total funding of $3.6bn in Q1, $2.6bn in Q2 and $2.9bn in Q3, said LSI’s Nick Talamantes. In 2026, he expects a continued investor focus on more mature firms in areas of oncology, cardiology and neurology.
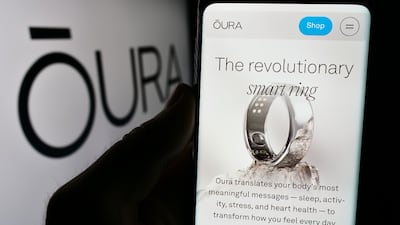
Brain-computer interfaces advance toward trials and commercialization, Oura pushes for FDA-cleared blood pressure monitoring, and regulators weigh AI’s expanding role in mental health and diagnostics amid rising safety concerns.
Data from Medicare patients shows no increased death risk for peripheral arterial disease patients treated with drug-coated devices, easing concerns that once led FDA to discourage use of the products. Researchers say the study could be a model for future large-scale cardiac device safety trials.
Viome’s CEO To Expand Into Clinical Diagnostics ‘For Which There Are No Solutions’ With AI, RNA Test
Viome Life Sciences accelerates its push into clinical diagnostics with studies in colorectal cancer and a major partnership with Microsoft to scale its molecular data analysis platform. Viome leverages RNA analysis and AI to detect disease at the molecular level and personalize preventive health.
US FDA inspections of three Philips manufacturing sites earlier this year resulted in a September warning letter that claimed the company was not in conformity with current good manufacturing practices. Philips says it is addressing the agency’s concerns and working to enhance its quality systems.
Wearables innovator Oura has launched a profile study aimed at helping the company secure FDA clearance for a blood pressure feature for its smart ring. Study participants will answer questions while wearing the ring, which the company plans to use to support the feature’s efficacy.