Financing
Women’s health start-up Midi Health has raised $100m in a Series D financing, led by Goodwater Capital with new investors Foresite Capital and Serena Ventures. Midi, which focuses on women in midlife, said the money will support the next phase of growth.
With capital being recycled, tariffs settling and interest rates softening, 2026 could provide more investment and growth opportunities in pharma and medtech, with AI giving a tailwind, say Taylor Wessing’s Ross McNaughton and Sarah Cole.
EIT Health has helped 3,000 start-ups and SMEs scale across Europe and supported over 120 innovations to launch. Inspired by the US MIT, the EIT Health program is on a mission to raise awareness of its value to healthtech innovators as it embarks on new methods of funding its own activities.
Atul Gupta, MD and CMO of Royal Philips’ Diagnosis & Treatment business, shares his views about potential developments in imaging and wider medtech in 2026 and the value of AI. He warns that clinicians will not adopt AI they don’t trust, understand or find helpful in real-world conditions.
Wishes for 2026 from our medtech experts focused on value-based care, faster reimbursements, and better collaboration. Key ideas included enhancing patient engagement, reducing waste, and aligning coverage policies to improve health outcomes and innovation.
Our experts said investor confidence in medtech is recovering as we head into 2026. Expect larger, selective fundraising and heightened M&A activity, focusing on innovative firms in neurology, cardiovascular health, and AI-driven technologies. Companies with solid strategies are favored.
Medtech leaders expect faster change in 2026 as AI integration speeds up across all business aspects, according to a Deloitte survey of medtech execs that also predicted M&A activity will remain strong in 2026. While optimism is high, uncertainties remain.
Neurotech start-up Subsense raised another $10m, bringing its total seed funding to $27m. The proceeds will be used primarily to accelerate and enhance the company’s pre-clinical research program.
Xeltis secured €50m to scale and market its aXess vascular access graft, a bioresorbable scaffold that transforms into a patient’s own living vessel. With EU regulatory review underway and US trials advancing, Xeltis sees new US Medicare rules as a major opening for alternative treatments.
Medtech funding in 2025 sees a trend of fewer, yet larger deals with total funding of $3.6bn in Q1, $2.6bn in Q2 and $2.9bn in Q3, said LSI’s Nick Talamantes. In 2026, he expects a continued investor focus on more mature firms in areas of oncology, cardiology and neurology.
Synchron raised $200m in series D funding to support pivotal trials in 2026 as well as commercialization of its Stentrode BCI. It also announced plans for a next-gen high-channel whole-brain interface and a new engineering hub in San Diego.
The neuromodulation space is quickly filling with well-funded companies that have demonstrated early signs of success. However, with strategics seemingly unwilling to spend on novel technologies and pharma desperate to maintain its foothold in CNS, these companies will face many challenges.
The $250m in new funding from HistoSonics’ new owners and new investors Thiel Bio and Founders Fund will expand its histotripsy platform globally, advance new indications, and scale operations. CEO Mike Blue tells Medtech Insight that an IPO isn’t an immediate priority.
Armor Medical’s wrist-worn wearable detected postpartum hemorrhage five times earlier than standard care in earlier studies. After surviving near-fatal hemorrhage herself, co-founder/CEO Kelsey Mayo aims to bring the device to market in 2028.
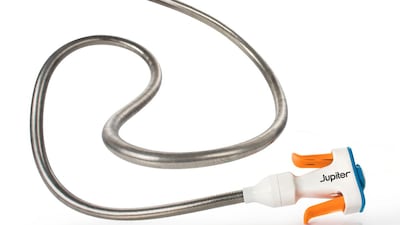
Jupiter Endovascular is positioning Vertex as a next-generation pulmonary thrombectomy solution that could redefine procedural standards, especially if ongoing SPIRARE trials confirm its clinical advantages.
J&J announced the separation of its orthopedics unit into a stand-alone company. The firm as a whole expects sales growth of over 5% in 2026. Gabelli analyst Jennie Tsai foresees more consolidation in orthopedics led by Zimmer Biomet, Stryker and DePuy as well as more acquisitions.
Family offices, impact funds, and public-private partnerships are among alternative approaches to financing that stress early-stage investment, collaboration, impact and health equity – but not, at least for family practices, AI.
Confido Health has raised $10m in Series A funding to expand its AI voice agent from patient scheduling into referrals, prescriptions and billing, bringing the total collected to $13m. CEO Chetan Reddy predicts in the next decade, AI use for admin tasks in healthcare will become mainstream.
While Sunrise’s main differentiator is a sensor that detects movement of the lower jaw, the company’s growth plan leans heavily on artificial intelligence to make care scalable. In practice, that means AI handles data triage, therapy monitoring and adherence support.
Sonomind’s immediate focus is treatment-resistant depression, but the company sees broader applications for its platform to treat psychiatric conditions such as anxiety, post-traumatic stress disorder and addiction.