The medtech giant violated USA and California antitrust law in its sales practices for devices used to cut and seal blood vessels during surgery, a jury found. Rival firm Applied Medical brought the case.
Stryker’s broader orthopedics portfolio continued to benefit from strong robotic demand during the quarter, with the company reporting 28.7% organic growth in its US Orthopedics other than hips and knees business.
Medtronic has agreed to acquire Cathworks for up to $585m, converting a long-standing partnership into full ownership as it expands its footprint in coronary physiology and AI-enabled cardiovascular tools.
The US FDA says Abiomed has reported 22 serious injuries linked to an issue with some of its temporary heart pumps that provide support to patients with acute right heart failure.
INBRAIN unveiled a bidirectional "rice-sized" BCI chip partnership, Merck commercialization progress and new speech-decoding trial in France as it advances its graphene-based cortical interface toward commercialization, pending regulatory clearance.
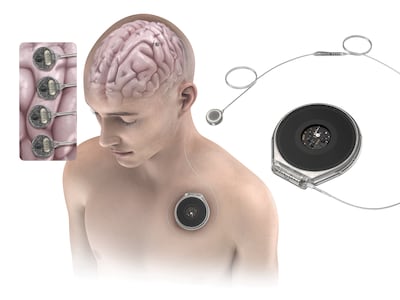
In this final part of a three-part series, Medtech Insight spoke with a neuroethicist and the first person in a trial using a BCI implant for stimulating hand movement. This story explores ethical considerations that arise when projects can no longer support patients with implanted devices.
The FDA has cleared Intuitive Surgical’s da Vinci 5 for some cardiac procedures, opening new opportunities for the firm. The company is also looking to expand its presence in ambulatory surgical centers, execs said during a Jan. 22 earnings call.
BioStem Technologies’ buyout of BioTissue Holding’s surgical and wound-care business adds cryopreserved and sterile technologies, Cryotek and SteriTek, and a direct sales force focused on acute care settings.
Abbott’s newly CE-marked TactiFlex Duo dual-ablation catheter used for treating AFib is competing against products already introduced by Boston Scientific, J&J and Medtronic.
The US FDA has issued an early alert concerning some Axios stent and delivery systems used in endoscopic drainage procedures after receiving reports of serious injuries and deaths linked to the device.
Amid the spectacle of humanoid robots at CES 2026, Swedish medical simulation company Surgical Science opted for a quieter pitch for its suitcase-sized robotic surgery simulator aimed at taking training out of the OR.
Johnson & Johnson is seeking marketing authorization from the US FDA for its Ottava surgical robot for upper abdomen procedures. If granted, Ottava could prove stiff competition for Medtronic and Intuitive.
The MAGiC catheter works with Stereotaxis’ robotic magnetic navigation technology, which allows physicians to steer catheters through the heart with precision and stability.
The US FDA’s General Hospital and Personal Use Devices Panel of the Medical Devices Advisory Committee convened Wednesday to discuss germicidal ultraviolet (GUV) devices as a mode of disinfection, a technology that has emerged since the COVID-19 pandemic.
Xeltis secured €50m to scale and market its aXess vascular access graft, a bioresorbable scaffold that transforms into a patient’s own living vessel. With EU regulatory review underway and US trials advancing, Xeltis sees new US Medicare rules as a major opening for alternative treatments.
The US FDA says Olympus has updated its instructions for a device used in many endoscopic procedures after reports of serious injuries. The class I recall follows the FDA blocking imports of other scoping devices from the Japanese firm earlier this year.
Medtronic won FDA clearance for its Hugo surgical robot for urologic procedures, which Wiliam Blair analyst expects will draw interest from physicians. But he also says that Intuitive Surgical will remain the clear dominant player.
Paradromics won FDA clearance to test its Connexus BCI in two people with severe speech impairment. The 2026 trial will assess whether the device can decode speech in real time.
Medtech Insight spoke with Rambam Medical Center’s Michael Mimouni, who implanted the first 3D-bioprinted corneal graft in a human patient, about his hopes for Precise Bio’s approach. The patient is part of a Phase I trial evaluating PB-001 in patients with corneal edema.
Brain-computer interfaces advance toward trials and commercialization, Oura pushes for FDA-cleared blood pressure monitoring, and regulators weigh AI’s expanding role in mental health and diagnostics amid rising safety concerns.