Archive
Editor’s note: This is your final call to participate in the survey to better understand our subscribers’ content and delivery needs. The deadline is 20 September.
Mary Jane Hinrichs, Ipsen’s head of early development, talks to In Vivo about getting ahead of the competition by securing deals for candidates before they enter Phase I trials.
Editor’s note: We are conducting a survey to better understand our subscribers’ content and delivery needs. If there are any changes you’d like to see in the coverage topics, content format or the method in which you receive and access In Vivo, or if you love it how it is, now is the time to have your voice heard.
The cell and gene therapy (CGT) clinical trial landscape in general and CAR-T cell clinical trials in particular are a special focus for the FDA, EMA, and other regulatory agencies. The whole industry is thus aware of the recent FDA safety investigation and requirements for labeling CAR therapy products.
Introducing the all-new Citeline News and Insights app, providing seamless access to all your favorite award-winning publications. Explore the latest articles from Pink Sheet, Scrip, In Vivo, Medtech Insight, Generics Bulletin and HBW Insight, all in one stylish, intuitive and user-friendly platform.
Tune in to this one-off podcast feature discussing the state-of-play in the diagnostics sector, with guests from ImmunExpress, Lucid Diagnostics and ChromaCode.
With the acquisition of Swiss biotech firm VectivBio in late June, Ironwood Pharmaceuticals has firmly positioned itself as a gastrointestinal-focused company.
Datamonitor Healthcare analysts discuss key data presentations from the American Society of Clinical Oncology's annual meeting, which took place in Chicago, 2-6 June.
Having led the way to regulatory approval for Kymriah and now Ebvallo, Atara CEO Pascal Touchon reflects on the evolution of cell therapy and what his company is doing differently.
The 2023 MedTech Forum attracted record interest from industry delegates in Europe and beyond. Two days of public sessions included the popular “CEOs No Filter” roundtables, where senior sector representatives were given carte blanche to appraise the state of Europe’s industry.
Citeline journalists report the top stories from the 2023 American Society of Clinical Oncology annual meeting, held in Chicago, US, 2-6 June.
Following the FDA’s release of draft guidance to enhance patient voice, In Vivo spoke with Ipsen’s rare disease regulation expert on creating a more patient-centric R&D and approval pathway, from modified endpoints to trial decentralization and beyond.
An interactive look at recent executive-level company changes and promotions in the biopharma, medical device and diagnostics industries.
An interactive look at recent executive-level company changes and promotions in the biopharma, medical device and diagnostics industries.
A selection of articles you may have missed from January 2023, covering the FDA's approach to gene therapies and their long-term implications, the rise (and possible fall) of Chinese companies' efforts in drug development and the ongoing consideration of the 'E' in ESG.
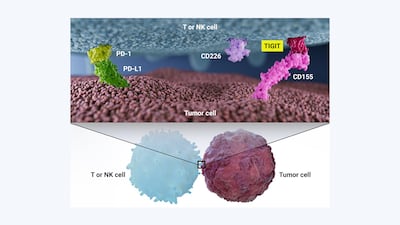
The promise of TIGIT as an oncology target in combination with PD-1/PD-L1 inhibitors has prompted an influx of investment into anti-TIGIT agents and five agents are already in advanced clinical trials. That initial enthusiasm has been dampened as of late, after disappointing Phase III data. Investors are hoping that 2023 will be the year that restores hope in the anti-TIGIT pipeline.
An interactive look at recent executive-level company changes and promotions in the biopharma, medical device and diagnostics industries.
With key milestones on the horizon for US biosimilars in 2022 and 2023, Cardinal Health’s vice president of biosimilars, Sonia Oskouei, explains how the industry can best position itself to take advantage of these upcoming opportunities – including potentially a global leadership role in ophthalmology. Oskouei discusses the importance of real-world evidence and education in ensuring confidence in biosimilars, as well as the ongoing struggle against misinformation and the need for clarity over concepts such as interchangeability and unbranded biologics.
Answer 10 minutes of questions about what you want from our stories and be eligible to win a €/£/$100 gift card.
For Biogen/Eisai’s success with Aduhelm, there were 93 failures in the anti-amyloid field. In Vivo's sister publication Scrip took a look back at how the pipeline for amyloid-targeting Alzheimer’s drugs progressed over the past decade to showcase exactly how difficult the Alzheimer’s space has been.