Clinical Trials
INBRAIN unveiled a bidirectional "rice-sized" BCI chip partnership, Merck commercialization progress and new speech-decoding trial in France as it advances its graphene-based cortical interface toward commercialization, pending regulatory clearance.
Medtech Insight was invited to moderate a panel discussion with leading experts in neuroscience and AI during INBRAIN’s five-year anniversary in Barcelona, Spain. Panelists discussed the promises, perils in BCI development, neuroethics and outlook.
In this final part of a three-part series, Medtech Insight spoke with a neuroethicist and the first person in a trial using a BCI implant for stimulating hand movement. This story explores ethical considerations that arise when projects can no longer support patients with implanted devices.
Johnson & Johnson is seeking marketing authorization from the US FDA for its Ottava surgical robot for upper abdomen procedures. If granted, Ottava could prove stiff competition for Medtronic and Intuitive.
The FDA’s revised guidance emphasizes biological sex in clinical trials, removing references to gender and health equity considerations.
The US FDA has made incorporating real-world evidence into medical device decisions a lot easier. The agency now says it will accept RWE without requiring it to contain identifiable individual patient data, making more of it available. Many stakeholders welcomed the change.
Shantanu Gaur, CEO of Allurion Technologies, a company that has developed a swallowable gastric balloon, believes that GLP-1 sales could indirectly lead to increased demand for medical device-based bariatric procedures, especially as patients seek alternatives to drug side effects and weight regain.
Neurotech start-up Subsense raised another $10m, bringing its total seed funding to $27m. The proceeds will be used primarily to accelerate and enhance the company’s pre-clinical research program.
The US FDA’s General Hospital and Personal Use Devices Panel of the Medical Devices Advisory Committee convened Wednesday to discuss germicidal ultraviolet (GUV) devices as a mode of disinfection, a technology that has emerged since the COVID-19 pandemic.
Aerogen, which is Ireland’s largest native medical technology firm, is in clinical trials for Aerofact, a nebulizer aimed at aiding premature infants' breathing. The technology promises easier administration of surfactant, with potential commercial rollout projected in three to four years, if succes
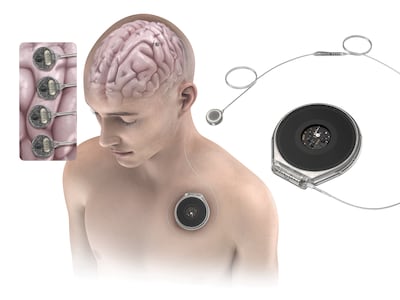
Paradromics won FDA clearance to test its Connexus BCI in two people with severe speech impairment. The 2026 trial will assess whether the device can decode speech in real time.
US healthcare innovation agency ARPA-H has announced a $100m initiative focused on building up the evidence base for fast-acting mental health treatments such as neuromodulation and digital therapeutics. The project is the first major initiative to be led by new ARPA-H director Alicia Jackson.
Medtech Insight spoke with Rambam Medical Center’s Michael Mimouni, who implanted the first 3D-bioprinted corneal graft in a human patient, about his hopes for Precise Bio’s approach. The patient is part of a Phase I trial evaluating PB-001 in patients with corneal edema.
BrainsWay advanced its Deep TMS platform with an FDA labeling expansion for adolescent MDD and the launch of a 200-patient alcohol use disorder trial. The company posted strong Q3 growth and is pursuing new indications and at-home neuromodulation through its Neuralief investment.
Data from Medicare patients shows no increased death risk for peripheral arterial disease patients treated with drug-coated devices, easing concerns that once led FDA to discourage use of the products. Researchers say the study could be a model for future large-scale cardiac device safety trials.
Neurotech start-up Subsense started proof-of-concept studies in mice hoping to show by late 2026 that its nonsurgical, nanoparticle-driven brain-computer interface can read and stimulate neural activity and treat conditions such as Parkinson’s and epilepsy.
INBRAIN teamed up with Microsoft to apply agentic AI to analyze real-time brain data and eventually recommend programming like a “mini-neurologist,” said CEO Carolina Aguilar. It also seeks to enable scalable deployment of INBRAIN’s graphene-based technology and potential joined research.
Synchron raised $200m in series D funding to support pivotal trials in 2026 as well as commercialization of its Stentrode BCI. It also announced plans for a next-gen high-channel whole-brain interface and a new engineering hub in San Diego.
Nearly one year into leading their agencies, US FDA Commission Marty Makary and CMS Administrator Mehmet Oz took to the stage together at the Milken Institute’s Future of Health Summit 2025 to highlight what they see as progress and lay out plans for the road ahead.
Advocates spanning the spectrum of women’s health met in Manhattan to discuss the gender disparities that remain in healthcare and how public policy can correct them and the enormous ROI investors in women’s health can potentially reap.