Market Access
Molecular diagnostics firm SAGA Diagnostics has introduced a colorectal cancer test that can detect even very low levels of cancer biomarkers. The test helps clinicians guide post-surgical treatment decisions.
Start-up medtech firms in the west of Ireland can benefit from incubator programs at two of the region’s universities, which support innovators across the medical device and digital health industries from concept to multi-employee businesses.
Entering the liquid biopsy market with a new technology remains challenging. Rarity Bioscience CEO Linus Bosaeus, is confident his company’s superRCA could help unlock the true potential of precision medicine as pharma looks more to minimal residual disease as an oncology endpoint.
Inspired by his son’s battle with cancer, Neal Piper founded Luminoah to modernize enteral feeding. Its smart, pocket-sized pump tracks nutrition in real time to prevent malnutrition and improve quality of life for patients and caregivers.
June Raine was a “hard act to follow” at MHRA, but new chief executive Lawrence Tallon is looking to the future in setting out a vision for the UK regulator.
The US Department of Commerce’s investigation into the national security impact of importing various medical equipment could lead to tariffs on many products. Device industry responds by emphasizing the robustness of domestic production, but also the rigidity of supply and reimbursement agreements.
At LSX World Congress USA, medtech executives shared lessons on global expansion, from regulatory pitfalls to cultural nuances and funding gaps. Their message: Prepare early, secure capital, and choose partners wisely.
Launching a medical device in Japan requires a solid reimbursement strategy as patients can't pay out of pocket. Companies must build ties with experts and prepare a comprehensive reimbursement dossier. Japan's strict pricing and focus on clinical benefits make early planning essential for success.
The latest report also demonstrates the EU’s trade relationships around the world, including with the US.
Medicare’s device coverage process is “a system in crisis,” consultant Bruce Quinn warned at the NextGen Dx Summit. He criticized bloated NCDs, stalled advisory committees, and years-long delays, urging CMS to streamline LCDs and adopt a six-month “fast-track” model to speed innovation access.
Signos links users to Dexcom’s Stelo for glucose readings and historic trends. The app is available in iOS and Android from the firm’s website, where it’s priced at $139 for three-month plan and $129 for a two-month plan, or online app stores.
The US FDA has announced its user fees for fiscal 2026, which are based on a figure of $455,000 for a PMA.
The US Department of Health and Human Services has proposed cutting Medicare reimbursement for skin substitutes used in chronic wound care by up to 90%. The change aims to address rising costs but could harm patient access and treatment quality, prompting concerns from industry stakeholders.
An “NHS Innovator Passport” that allows quicker uptake of proven technologies across the national healthcare provider is one of the wins the UK government is looking to score from its new Life Sciences Sector plan. The plan majors on prevention. The medtech industry has given it a cautious welcome.
The pulsed-field ablation market is surging, with the UK NHS opening doors and FDA updates for major players. Medtech Insight spoke with Steven Mickelsen, founder of Farapulse (the first clinically approved PFA system), about the sector's growth and his new venture, Field Medical.
The NHS 10-Year Plan officially released on July 3 will crystalize NICE’s Rules-Based Pathway – a new concept for medtech evaluation that will come with a guarantee of funding – but only for a select number of products. Government to also introduce “innovator passport” to speed innovation into NHS.
Legal experts warn, however, that new Mexican procurement rules are discriminatory and could be challenged in the courts.
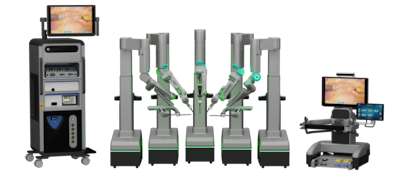
SS Innovations reported its first SSi Mantra 3 robotic cardiac surgery in South America as it prepares to file for US FDA de novo clearance and a CE mark. The firm aims to challenge Intuitive Surgical with a lower-cost, flexible system targeting US and European markets.
Market access concerns dominated at the annual BioWales In London event, where AstraZeneca set out industry’s priorities and the UK Office for Life Sciences struck an optimistic tone about how the UK will lastingly improve uptake of innovation.
Annual survey of patients and professionals shows how attitudes to health system transformation are evolving and what stakeholders are demanding as acceptance of AI tools accelerates.